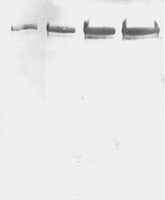
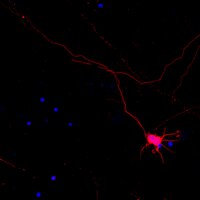
Hippocampal Synaptic Expansion Induced by Spatial Experience in Rats Correlates with Improved Information Processing in the Hippocampus.
Carasatorre, M; Ochoa-Alvarez, A; Velázquez-Campos, G; Lozano-Flores, C; Ramírez-Amaya, V; Díaz-Cintra, SY
PloS one
10
e0132676
2015
Show Abstract
Spatial water maze (WM) overtraining induces hippocampal mossy fiber (MF) expansion, and it has been suggested that spatial pattern separation depends on the MF pathway. We hypothesized that WM experience inducing MF expansion in rats would improve spatial pattern separation in the hippocampal network. We first tested this by using the the delayed non-matching to place task (DNMP), in animals that had been previously trained on the water maze (WM) and found that these animals, as well as animals treated as swim controls (SC), performed better than home cage control animals the DNMP task. The "catFISH" imaging method provided neurophysiological evidence that hippocampal pattern separation improved in animals treated as SC, and this improvement was even clearer in animals that experienced the WM training. Moreover, these behavioral treatments also enhance network reliability and improve partial pattern separation in CA1 and pattern completion in CA3. By measuring the area occupied by synaptophysin staining in both the stratum oriens and the stratun lucidum of the distal CA3, we found evidence of structural synaptic plasticity that likely includes MF expansion. Finally, the measures of hippocampal network coding obtained with catFISH correlate significantly with the increased density of synaptophysin staining, strongly suggesting that structural synaptic plasticity in the hippocampus induced by the WM and SC experience is related to the improvement of spatial information processing in the hippocampus. | | | 26244549
 |
Highly efficient direct conversion of human fibroblasts to neuronal cells by chemical compounds.
Dai, P; Harada, Y; Takamatsu, T
Journal of clinical biochemistry and nutrition
56
166-70
2015
Show Abstract
Direct conversion of mammalian fibroblasts into induced neuronal (iN) cells has been attained by forced expression of pro-neural transcriptional factors, or by combining defined factors with either microRNAs or small molecules. Here, we show that neuronal cells can be converted from postnatal human fibroblasts into cell populations with neuronal purities of up to greater than 80% using a combination of six chemical compounds. The chemical compound-induced neuronal cells (CiNCs) express neuron-specific proteins and functional neuron markers. The efficiency of CiNCs is unaffected by either the donor's age or cellular senescence (passage number). We propose this chemical direct converting strategy as a potential approach for highly efficient generation of neuronal cells from human fibroblasts for such uses as in neural disease modeling and regenerative medicine. | | | 26060345
 |
Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity.
You, X; Vlatkovic, I; Babic, A; Will, T; Epstein, I; Tushev, G; Akbalik, G; Wang, M; Glock, C; Quedenau, C; Wang, X; Hou, J; Liu, H; Sun, W; Sambandan, S; Chen, T; Schuman, EM; Chen, W
Nature neuroscience
18
603-10
2015
Show Abstract
Circular RNAs (circRNAs) have re-emerged as an interesting RNA species. Using deep RNA profiling in different mouse tissues, we observed that circRNAs were substantially enriched in brain and a disproportionate fraction of them were derived from host genes that encode synaptic proteins. Moreover, on the basis of separate profiling of the RNAs localized in neuronal cell bodies and neuropil, circRNAs were, on average, more enriched in the neuropil than their host gene mRNA isoforms. Using high-resolution in situ hybridization, we visualized circRNA punctae in the dendrites of neurons. Consistent with the idea that circRNAs might regulate synaptic function during development, many circRNAs changed their abundance abruptly at a time corresponding to synaptogenesis. In addition, following a homeostatic downscaling of neuronal activity many circRNAs exhibited substantial up- or downregulation. Together, our data indicate that brain circRNAs are positioned to respond to and regulate synaptic function. | | | 25714049
 |
Interaction between neural stem cells and bone marrow derived-mesenchymal stem cells during differentiation.
Rong, JU; Wen, Z; Rong, WU; Zhichun, F
Biomedical reports
3
242-246
2015
Show Abstract
Due to their capacity to self-replicate or produce specific differentiated cell types, neural stem cells (NSCs) and bone marrow derived-mesenchymal stem cells (BMSCs) are potential sources for cell transplantation therapies, particularly for neural injury. However, the interaction between NSCs and BMSCs during differentiation has not yet been defined. The interaction is believed to improve the effectiveness and efficiency of cell therapy. In the present study, human NSCs and BMSCs were cultured and the Transwell co-culture system was used to observe the interplay between NSCs and BMSCs during differentiation. The results revealed that NSCs promoted BMSCs to differentiate into neurons and NSCs; whereas, BMSCs did not affect the differentiation of NSCs. Simultaneously, co-culture increased the concentration of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which are secreted by NSCs and BMSCs. The present findings suggest that co-culture of NSCs and BMSCs can promote the differentiation and this process may be modulated by BDNF and NGF. | | | 25798249
 |
NMDA receptor GluN2A/GluN2B subunit ratio as synaptic trait of levodopa-induced dyskinesias: from experimental models to patients.
Mellone, M; Stanic, J; Hernandez, LF; Iglesias, E; Zianni, E; Longhi, A; Prigent, A; Picconi, B; Calabresi, P; Hirsch, EC; Obeso, JA; Di Luca, M; Gardoni, F
Frontiers in cellular neuroscience
9
245
2015
Show Abstract
Levodopa-induced dyskinesias (LIDs) are major complications in the pharmacological management of Parkinson's disease (PD). Abnormal glutamatergic transmission in the striatum is considered a key factor in the development of LIDs. This work aims at: (i) characterizing N-methyl-D-aspartate (NMDA) receptor GluN2A/GluN2B subunit ratio as a common synaptic trait in rat and primate models of LIDs as well as in dyskinetic PD patients; and (ii) validating the potential therapeutic effect of a cell-permeable peptide (CPP) interfering with GluN2A synaptic localization on the dyskinetic behavior of these experimental models of LIDs. Here we demonstrate an altered ratio of synaptic GluN2A/GluN2B-containing NMDA receptors in the striatum of levodopa-treated dyskinetic rats and monkeys as well as in post-mortem tissue from dyskinetic PD patients. The modulation of synaptic NMDA receptor composition by a cell-permeable peptide interfering with GluN2A subunit interaction with the scaffolding protein postsynaptic density protein 95 (PSD-95) leads to a reduction in the dyskinetic motor behavior in the two animal models of LIDs. Our results indicate that targeting synaptic NMDA receptor subunit composition may represent an intriguing therapeutic approach aimed at ameliorating levodopa motor side effects. | | | 26217176
 |
Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS.
Franssen, EH; Zhao, RR; Koseki, H; Kanamarlapudi, V; Hoogenraad, CC; Eva, R; Fawcett, JW
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
8359-75
2015
Show Abstract
Integrins are adhesion and survival molecules involved in axon growth during CNS development, as well as axon regeneration after injury in the peripheral nervous system (PNS). Adult CNS axons do not regenerate after injury, partly due to a low intrinsic growth capacity. We have previously studied the role of integrins in axon growth in PNS axons; in the present study, we investigate whether integrin mechanisms involved in PNS regeneration may be altered or lacking from mature CNS axons by studying maturing CNS neurons in vitro. In rat cortical neurons, we find that integrins are present in axons during initial growth but later become restricted to the somato-dendritic domain. We investigated how this occurs and whether it can be altered to enhance axonal growth potential. We find a developmental change in integrin trafficking; transport becomes predominantly retrograde throughout axons, but not dendrites, as neurons mature. The directionality of transport is controlled through the activation state of ARF6, with developmental upregulation of the ARF6 GEF ARNO enhancing retrograde transport. Lowering ARF6 activity in mature neurons restores anterograde integrin flow, allows transport into axons, and increases axon growth. In addition, we found that the axon initial segment is partly responsible for exclusion of integrins and removal of this structure allows integrins into axons. Changing posttranslational modifications of tubulin with taxol also allows integrins into the proximal axon. The experiments suggest that the developmental loss of regenerative ability in CNS axons is due to exclusion of growth-related molecules due to changes in trafficking. | | | 26019348
 |
Properties of neurons derived from induced pluripotent stem cells of Gaucher disease type 2 patient fibroblasts: potential role in neuropathology.
Sun, Y; Florer, J; Mayhew, CN; Jia, Z; Zhao, Z; Xu, K; Ran, H; Liou, B; Zhang, W; Setchell, KD; Gu, J; Grabowski, GA
PloS one
10
e0118771
2015
Show Abstract
Gaucher disease (GD) is caused by insufficient activity of acid β-glucosidase (GCase) resulting from mutations in GBA1. To understand the pathogenesis of the neuronopathic GD, induced pluripotent stem cells (iPSCs) were generated from fibroblasts isolated from three GD type 2 (GD2) and 2 unaffected (normal and GD carrier) individuals. The iPSCs were converted to neural precursor cells (NPCs) which were further differentiated into neurons. Parental GD2 fibroblasts as well as iPSCs, NPCs, and neurons had similar degrees of GCase deficiency. Lipid analyses showed increases of glucosylsphingosine and glucosylceramide in the GD2 cells. In addition, GD2 neurons showed increased α-synuclein protein compared to control neurons. Whole cell patch-clamping of the GD2 and control iPSCs-derived neurons demonstrated excitation characteristics of neurons, but intriguingly, those from GD2 exhibited consistently less negative resting membrane potentials with various degree of reduction in action potential amplitudes, sodium and potassium currents. Culture of control neurons in the presence of the GCase inhibitor (conduritol B epoxide) recapitulated these findings, providing a functional link between decreased GCase activity in GD and abnormal neuronal electrophysiological properties. To our knowledge, this study is first to report abnormal electrophysiological properties in GD2 iPSC-derived neurons that may underlie the neuropathic phenotype in Gaucher disease. | Immunofluorescence | | 25822147
 |
Convection-enhanced delivery of AAV2-PrPshRNA in prion-infected mice.
Ahn, M; Bajsarowicz, K; Oehler, A; Lemus, A; Bankiewicz, K; DeArmond, SJ
PloS one
9
e98496
2014
Show Abstract
Prion disease is caused by a single pathogenic protein (PrPSc), an abnormal conformer of the normal cellular prion protein PrPC. Depletion of PrPC in prion knockout mice makes them resistant to prion disease. Thus, gene silencing of the Prnp gene is a promising effective therapeutic approach. Here, we examined adeno-associated virus vector type 2 encoding a short hairpin RNA targeting Prnp mRNA (AAV2-PrP-shRNA) to suppress PrPC expression both in vitro and in vivo. AAV2-PrP-shRNA treatment suppressed PrP levels and prevented dendritic degeneration in RML-infected brain aggregate cultures. Infusion of AAV2-PrP-shRNA-eGFP into the thalamus of CD-1 mice showed that eGFP was transported to the cerebral cortex via anterograde transport and the overall PrPC levels were reduced by ∼ 70% within 4 weeks. For therapeutic purposes, we treated RML-infected CD-1 mice with AAV2-PrP-shRNA beginning at 50 days post inoculation. Although AAV2-PrP-shRNA focally suppressed PrPSc formation in the thalamic infusion site by ∼ 75%, it did not suppress PrPSc formation efficiently in other regions of the brain. Survival of mice was not extended compared to the untreated controls. Global suppression of PrPC in the brain is required for successful therapy of prion diseases. | | | 24866748
 |
Synaptic function of nicastrin in hippocampal neurons.
Lee, SH; Sharma, M; Südhof, TC; Shen, J
Proceedings of the National Academy of Sciences of the United States of America
111
8973-8
2014
Show Abstract
Synaptic dysfunction is widely thought to play a key role in the pathogenesis of Alzheimer's disease (AD). Presenilins, the major gene products involved in familial AD, are essential for short- and long-term synaptic plasticity in mature neurons as well as for the survival of cortical neurons during aging. Presenilin and nicastrin are both indispensable components of the γ-secretase complex, but it remains unknown whether presenilin regulates synaptic function in a γ-secretase-dependent or γ-secretase-independent manner and whether nicastrin plays similar roles in central synapses. In the current study, we address these questions using an electrophysiological approach to analyze nicastrin conditional knockout (cKO) mice in the hippocampal Schaffer collateral pathway. In these mice, we found that, even at 2 mo of age, deletion of nicastrin in excitatory neurons of the postnatal forebrain using Cre recombinase expressed under the control of the αCaMKII promoter led to deficits in presynaptic short-term plasticity including paired-pulse facilitation and frequency facilitation. Depletion of Ca(2+) in the endoplasmic reticulum mimics and occludes the presynaptic facilitation deficits in nicastrin cKO mice, suggesting that disrupted intracellular Ca(2+) homeostasis underlies the presynaptic deficits. In addition, NMDA receptor-mediated responses and long-term potentiation induced by theta-burst stimulation were decreased in nicastrin cKO mice at 3 mo but not at 2 mo of age. Together, these findings show that, similar to presenilins, nicastrin plays essential roles in the regulation of short- and long-term synaptic plasticity, highlighting the importance of γ-secretase in the function of mature synapses. | Western Blotting | Mouse | 24889619
 |
A single-cell and feeder-free culture system for monkey embryonic stem cells.
Ono, T; Suzuki, Y; Kato, Y; Fujita, R; Araki, T; Yamashita, T; Kato, H; Torii, R; Sato, N
PloS one
9
e88346
2014
Show Abstract
Primate pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), hold great potential for research and application in regenerative medicine and drug discovery. To maximize primate PSC potential, a practical system is required for generating desired functional cells and reproducible differentiation techniques. Much progress regarding their culture systems has been reported to date; however, better methods would still be required for their practical use, particularly in industrial and clinical fields. Here we report a new single-cell and feeder-free culture system for primate PSCs, the key feature of which is an originally formulated serum-free medium containing FGF and activin. In this culture system, cynomolgus monkey ESCs can be passaged many times by single-cell dissociation with traditional trypsin treatment and can be propagated with a high proliferation rate as a monolayer without any feeder cells; further, typical PSC properties and genomic stability can be retained. In addition, it has been demonstrated that monkey ESCs maintained in the culture system can be used for various experiments such as in vitro differentiation and gene manipulation. Thus, compared with the conventional culture system, monkey ESCs grown in the aforementioned culture system can serve as a cell source with the following practical advantages: simple, stable, and easy cell maintenance; gene manipulation; cryopreservation; and desired differentiation. We propose that this culture system can serve as a reliable platform to prepare primate PSCs useful for future research and application. | | | 24505480
 |