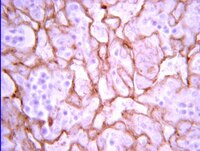
Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion.
Ishii, T; Miyazawa, M; Takanashi, Y; Tanigawa, M; Yasuda, K; Onouchi, H; Kawabe, N; Mitsushita, J; Hartman, PS; Ishii, N
Redox biology
2
679-85
2014
Show Abstract
Historical data in the 1950s suggests that 7%, 11%, 33%, and 87% of couples were infertile by ages 30, 35, 40 and 45, respectively. Up to 22.3% of infertile couples have unexplained infertility. Oxidative stress is associated with male and female infertility. However, there is insufficient evidence relating to the influence of oxidative stress on the maintenance of a viable pregnancy, including pregnancy complications and fetal development. Recently, we have established Tet-mev-1 conditional transgenic mice, which can express the doxycycline-induced mutant SDHC(V69E) transgene and experience mitochondrial respiratory chain dysfunction leading to intracellular oxidative stress. In this report, we demonstrate that this kind of abnormal mitochondrial respiratory chain-induced chronic oxidative stress affects fertility, pregnancy and delivery rates as well as causes recurrent abortions, occasionally resulting in maternal death. Despite this, spermatogenesis and early embryogenesis are completely normal, indicating the mutation's effects to be rather subtle. Female Tet-mev-1 mice exhibit thrombocytosis and splenomegaly in both non-pregnant and pregnant mice as well as placental angiodysplasia with reduced Flt-1 protein leading to hypoxic conditions, which could contribute to placental inflammation and fetal abnormal angiogenesis. Collectively these data strongly suggest that chronic oxidative stress caused by mitochondrial mutations provokes spontaneous abortions and recurrent miscarriage resulting in age-related female infertility. | 24936442
 |
Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy.
Dorrell, MI; Aguilar, E; Jacobson, R; Trauger, SA; Friedlander, J; Siuzdak, G; Friedlander, M
Glia
58
43-54
2010
Show Abstract
Astrocytes are well known modulators of normal developmental retinal vascularization. However, relatively little is known about the role of glial cells during pathological retinal neovascularization (NV), a leading contributor to vision loss in industrialized nations. We demonstrate that the loss of astrocytes and microglia directly correlates with the development of pathological NV in a mouse model of oxygen-induced retinopathy (OIR). These two distinct glial cell populations were found to have cooperative survival effects in vitro and in vivo. The intravitreal injection of myeloid progenitor cells, astrocytes, or astrocyte-conditioned media rescued endogenous astrocytes from degeneration that normally occurs within the hypoxic, vaso-obliterated retina following return to normoxia. Protection of the retinal astrocytes and microglia was directly correlated with accelerated revascularization of the normal retinal plexuses and reduction of pathological intravitreal NV normally associated with OIR. Using astrocyte-conditioned media, several factors were identified that may contribute to the observed astrocytic protection and subsequent normalization of the retinal vasculature, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Injection of VEGF or bFGF at specific doses rescued the retinas from developing OIR-associated pathology, an effect that was also preceded by protection of endogenous glia from hypoxia-induced degeneration. Together, these data suggest that vascular-associated glia are also required for normalized revascularization of the hypoxic retina. Methods developed to target and protect glial cells may provide a novel strategy by which normalized revascularization can be promoted and the consequences of abnormal NV in retinal vascular diseases can be prevented. | 19544395
 |
Temporal profile of angiogenesis and expression of related genes in the brain after ischemia.
Takeshi Hayashi, Nobuo Noshita, Taku Sugawara, Pak H Chan
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism
23
166-80
2003
Show Abstract
Angiogenesis is an intricately regulated phenomenon. Its mechanisms in the ischemic brain have not been clearly elucidated. The authors investigated expression of angiogenesis-related genes using a complementary DNA (cDNA) array method as well as Western blotting and immunohistochemistry, and compared these studies with a temporal profile of angiogenesis in mouse brains after ischemia. The number of vessels significantly increased 3 days after injury, and proliferating endothelial cells increased as early as 1 day. This means that angiogenesis occurs immediately after the injury. Ninety-six genes implicated in angiogenesis were investigated with a cDNA array study. It was found that 42, 29, and 13 genes were increased at 1 hour, 1 day, and 21 days, respectively. Most of the well-known angiogenic factors increased as early as 1 hour. Vessel-stabilizing factors such as thrombospondins also increased. At 1 day, however, thrombospondins decreased to lower levels than in the control, indicating a shift from vascular protection to angiogenesis. At 21 days, many genes were decreased, but some involved in tissue repair were newly increased. Western blotting and immunohistochemistry showed findings compatible with the cDNA array study. Many molecules act in an orchestrated fashion in the brain after ischemia and should be taken into account for therapeutic angiogenesis for stroke. | 12571448
 |