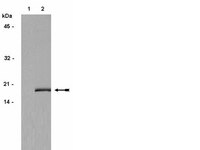
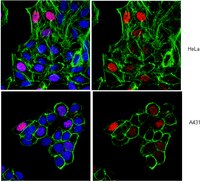
Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence.
Otsuki L, Brand AH
Science, 360(6384):99-102
2018
Show Abstract
Quiescent stem cells in adult tissues can be activated for homeostasis or repair. Neural stem cells (NSCs) in Drosophila are reactivated from quiescence in response to nutrition by the insulin signaling pathway. It is widely accepted that quiescent stem cells are arrested in G0 In this study, however, we demonstrate that quiescent NSCs (qNSCs) are arrested in either G2 or G0 G2-G0 heterogeneity directs NSC behavior: G2 qNSCs reactivate before G0 qNSCs. In addition, we show that the evolutionarily conserved pseudokinase Tribbles (Trbl) induces G2 NSCs to enter quiescence by promoting degradation of Cdc25String and that it subsequently maintains quiescence by inhibiting Akt activation. Insulin signaling overrides repression of Akt and silences trbl transcription, allowing NSCs to exit quiescence. Our results have implications for identifying and manipulating quiescent stem cells for regenerative purposes. | | | 29622651
 |
Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish.
Evason, KJ; Francisco, MT; Juric, V; Balakrishnan, S; Lopez Pazmino, Mdel P; Gordan, JD; Kakar, S; Spitsbergen, J; Goga, A; Stainier, DY
PLoS genetics
11
e1005305
2015
Show Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal human cancers. The search for targeted treatments has been hampered by the lack of relevant animal models for the genetically diverse subsets of HCC, including the 20-40% of HCCs that are defined by activating mutations in the gene encoding β-catenin. To address this chemotherapeutic challenge, we created and characterized transgenic zebrafish expressing hepatocyte-specific activated β-catenin. By 2 months post fertilization (mpf), 33% of transgenic zebrafish developed HCC in their livers, and 78% and 80% of transgenic zebrafish showed HCC at 6 and 12 mpf, respectively. As expected for a malignant process, transgenic zebrafish showed significantly decreased mean adult survival compared to non-transgenic control siblings. Using this novel transgenic model, we screened for druggable pathways that mediate β-catenin-induced liver growth and identified two c-Jun N-terminal kinase (JNK) inhibitors and two antidepressants (one tricyclic antidepressant, amitriptyline, and one selective serotonin reuptake inhibitor) that suppressed this phenotype. We further found that activated β-catenin was associated with JNK pathway hyperactivation in zebrafish and in human HCC. In zebrafish larvae, JNK inhibition decreased liver size specifically in the presence of activated β-catenin. The β-catenin-specific growth-inhibitory effect of targeting JNK was conserved in human liver cancer cells. Our other class of hits, antidepressants, has been used in patient treatment for decades, raising the exciting possibility that these drugs could potentially be repurposed for cancer treatment. In support of this proposal, we found that amitriptyline decreased tumor burden in a mouse HCC model. Our studies implicate JNK inhibitors and antidepressants as potential therapeutics for β-catenin-induced liver tumors. | | | 26134322
 |
Midostaurin preferentially attenuates proliferation of triple-negative breast cancer cell lines through inhibition of Aurora kinase family.
Kawai, M; Nakashima, A; Kamada, S; Kikkawa, U
Journal of biomedical science
22
48
2015
Show Abstract
Breast cancer is classified into three subtypes by the expression of biomarker receptors such as hormone receptors and human epidermal growth factor receptor 2. Triple-negative breast cancer (TNBC) expresses none of these receptors and has an aggressive phenotype with a poor prognosis, which is insensitive to the drugs that target the hormone receptors and human epidermal growth factor receptor 2. It is, thus, required to develop an effective therapeutic reagent to treat TNBC.The study using a panel of 19 breast cancer cell lines revealed that midostaurin, a multi-target protein kinase inhibitor, suppresses preferentially the growth of TNBC cells comparing with non-TNBC cells. Clustering analysis of the drug activity data for the panel of cancer cell lines predicted that midostaurin shares the target with Aurora kinase inhibitors. Following studies indicated that midostaurin attenuates the phosphorylation reaction mediated by Aurora kinase in the cells and directly inhibits this protein kinase in vitro, and that this reagent induces apoptosis accompanying accumulation of 4N and 8N DNA cells in TNBC cells.Midostaurin suppresses the proliferation of TNBC cells among the breast cancer cell lines presumably through the inhibition of the Aurora kinase family. The precise study of midostaurin on cell growth will contribute to the development of the drug for the treatment of TNBC. | | | 26141684
 |
TD-60 links RalA GTPase function to the CPC in mitosis.
Papini, D; Langemeyer, L; Abad, MA; Kerr, A; Samejima, I; Eyers, PA; Jeyaprakash, AA; Higgins, JM; Barr, FA; Earnshaw, WC
Nature communications
6
7678
2015
Show Abstract
TD-60 (also known as RCC2) is a highly conserved protein that structurally resembles the Ran guanine exchange factor (GEF) RCC1, but has not previously been shown to have GEF activity. TD-60 has a typical chromosomal passenger complex (CPC) distribution in mitotic cells, but associates with integrin complexes and is involved in cell motility during interphase. Here we show that TD-60 exhibits GEF activity, in vitro and in cells, for the small GTPase RalA. TD-60 or RalA depletion causes spindle abnormalities in prometaphase associated with abnormal centromeric accumulation of CPC components. TD-60 and RalA apparently work together to contribute to the regulation of kinetochore-microtubule interactions in early mitosis. Importantly, several mitotic phenotypes caused by TD-60 depletion are reverted by the expression of a GTP-locked mutant, RalA (Q72L). The demonstration that a small GTPase participates in the regulation of the CPC reveals a level of mitotic regulation not suspected in previous studies. | | | 26158537
 |
Expression of Hox, Cdx, and Six3/6 genes in the hoplonemertean Pantinonemertes californiensis offers insight into the evolution of maximally indirect development in the phylum Nemertea.
Hiebert, LS; Maslakova, SA
EvoDevo
6
26
2015
Show Abstract
Maximally indirect development via a pilidium larva is unique to the pilidiophoran clade of phylum Nemertea. All other nemerteans have more or less direct development. The origin of pilidial development with disjunct invaginated juvenile rudiments and catastrophic metamorphosis remains poorly understood. While basal members of the phylum, the Palaeonemertea, do not appear to have ever had a pilidium, certain similarity exists in the development of the Pilidiophora and the sister clade, the Hoplonemertea. It is unclear whether this similarity represents the homology and whether pilidial development evolved before or after pilidiophorans diverged from hoplonemerteans. To gain insight into these questions, we examined the expression of Hox, Cdx, and Six3/6 genes in the development of the hoplonemertean Pantinonemertes californiensis and expression of Six3/6 in the pilidium of Micrura alaskensis. To further characterize the function of larval structures showing expression of these genes, we examined the serotonergic nervous system and cell proliferation in P. californiensis.We show that Hox and Cdx genes, which pattern the pilidial imaginal discs giving rise to the juvenile trunk, are expressed in paired posterior epidermal invaginations in P. californiensis larvae. We also show that Six3/6 patterns both the pilidial cephalic discs, which give rise to the juvenile head, and a pair of anterior epidermal invaginations in hoplonemertean development. We show that anterior invaginations in larval P. californiensis are associated with a pair of serotonergic neurons, and thus may have a role in the development of the juvenile nervous system. This is similar to the role of cephalic discs in pilidiophoran development. Finally, we show that four zones of high cell proliferation correspond to the paired invaginations in P. californiensis, suggesting that these invaginations may play a similar role in the development of the hoplonemertean juvenile to the role of imaginal discs in the pilidium, which also exhibit high rates of cell proliferation.Expression of Hox, Cdx, and Six3/6 genes supports the homology between the imaginal discs of the pilidium and the paired larval invaginations in hoplonemerteans. This suggests that invaginated juvenile rudiments (possible precursors to pilidial imaginal discs) may have been present in the most recent common ancestor of the Pilidiophora and Hoplonemertea. | | | 26244086
 |
Sustained Pax6 Expression Generates Primate-like Basal Radial Glia in Developing Mouse Neocortex.
Wong, FK; Fei, JF; Mora-Bermúdez, F; Taverna, E; Haffner, C; Fu, J; Anastassiadis, K; Stewart, AF; Huttner, WB
PLoS biology
13
e1002217
2015
Show Abstract
The evolutionary expansion of the neocortex in mammals has been linked to enlargement of the subventricular zone (SVZ) and increased proliferative capacity of basal progenitors (BPs), notably basal radial glia (bRG). The transcription factor Pax6 is known to be highly expressed in primate, but not mouse, BPs. Here, we demonstrate that sustaining Pax6 expression selectively in BP-genic apical radial glia (aRG) and their BP progeny of embryonic mouse neocortex suffices to induce primate-like progenitor behaviour. Specifically, we conditionally expressed Pax6 by in utero electroporation using a novel, Tis21-CreERT2 mouse line. This expression altered aRG cleavage plane orientation to promote bRG generation, increased cell-cycle re-entry of BPs, and ultimately increased upper-layer neuron production. Upper-layer neuron production was also increased in double-transgenic mouse embryos with sustained Pax6 expression in the neurogenic lineage. Strikingly, increased BPs existed not only in the SVZ but also in the intermediate zone of the neocortex of these double-transgenic mouse embryos. In mutant mouse embryos lacking functional Pax6, the proportion of bRG among BPs was reduced. Our data identify specific Pax6 effects in BPs and imply that sustaining this Pax6 function in BPs could be a key aspect of SVZ enlargement and, consequently, the evolutionary expansion of the neocortex. | | | 26252244
 |
The lethal response to Cdk1 inhibition depends on sister chromatid alignment errors generated by KIF4 and isoform 1 of PRC1.
Voets, E; Marsman, J; Demmers, J; Beijersbergen, R; Wolthuis, R
Scientific reports
5
14798
2015
Show Abstract
Cyclin-dependent kinase 1 (Cdk1) is absolutely essential for cell division. Complete ablation of Cdk1 precludes the entry of G2 phase cells into mitosis, and is early embryonic lethal in mice. Dampening Cdk1 activation, by reducing gene expression or upon treatment with cell-permeable Cdk1 inhibitors, is also detrimental for proliferating cells, but has been associated with defects in mitotic progression, and the formation of aneuploid daughter cells. Here, we used a large-scale RNAi screen to identify the human genes that critically determine the cellular toxicity of Cdk1 inhibition. We show that Cdk1 inhibition leads to fatal sister chromatid alignment errors and mitotic arrest in the spindle checkpoint. These problems start early in mitosis and are alleviated by depletion of isoform 1 of PRC1 (PRC1-1), by gene ablation of its binding partner KIF4, or by abrogation of KIF4 motor activity. Our results show that, normally, Cdk1 activity must rise above the level required for mitotic entry. This prevents KIF4-dependent PRC1-1 translocation to astral microtubule tips and safeguards proper chromosome congression. We conclude that cell death in response to Cdk1 inhibitors directly relates to chromosome alignment defects generated by insufficient repression of PRC1-1 and KIF4 during prometaphase. | | | 26423135
 |
In vitro alterations do not reflect a requirement for host cell cycle progression during Plasmodium liver stage infection.
Hanson, KK; March, S; Ng, S; Bhatia, SN; Mota, MM
Eukaryotic cell
14
96-103
2015
Show Abstract
Prior to invading nonreplicative erythrocytes, Plasmodium parasites undergo their first obligate step in the mammalian host inside hepatocytes, where each sporozoite replicates to generate thousands of merozoites. While normally quiescent, hepatocytes retain proliferative capacity and can readily reenter the cell cycle in response to diverse stimuli. Many intracellular pathogens, including protozoan parasites, manipulate the cell cycle progression of their host cells for their own benefit, but it is not known whether the hepatocyte cell cycle plays a role during Plasmodium liver stage infection. Here, we show that Plasmodium parasites can be observed in mitotic hepatoma cells throughout liver stage development, where they initially reduce the likelihood of mitosis and ultimately lead to significant acquisition of a binucleate phenotype. However, hepatoma cells pharmacologically arrested in S phase still support robust and complete Plasmodium liver stage development, which thus does not require cell cycle progression in the infected cell in vitro. Furthermore, murine hepatocytes remain quiescent throughout in vivo infection with either Plasmodium berghei or Plasmodium yoelii, as do Plasmodium falciparum-infected primary human hepatocytes, demonstrating that the rapid and prodigious growth of liver stage parasites is accomplished independent of host hepatocyte cell cycle progression during natural infection. | | | 25416236
 |
Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury.
Vaughan, AE; Brumwell, AN; Xi, Y; Gotts, JE; Brownfield, DG; Treutlein, B; Tan, K; Tan, V; Liu, FC; Looney, MR; Matthay, MA; Rock, JR; Chapman, HA
Nature
517
621-5
2015
Show Abstract
Broadly, tissue regeneration is achieved in two ways: by proliferation of common differentiated cells and/or by deployment of specialized stem/progenitor cells. Which of these pathways applies is both organ- and injury-specific. Current models in the lung posit that epithelial repair can be attributed to cells expressing mature lineage markers. By contrast, here we define the regenerative role of previously uncharacterized, rare lineage-negative epithelial stem/progenitor (LNEP) cells present within normal distal lung. Quiescent LNEPs activate a ΔNp63 (a p63 splice variant) and cytokeratin 5 remodelling program after influenza or bleomycin injury in mice. Activated cells proliferate and migrate widely to occupy heavily injured areas depleted of mature lineages, at which point they differentiate towards mature epithelium. Lineage tracing revealed scant contribution of pre-existing mature epithelial cells in such repair, whereas orthotopic transplantation of LNEPs, isolated by a definitive surface profile identified through single-cell sequencing, directly demonstrated the proliferative capacity and multipotency of this population. LNEPs require Notch signalling to activate the ΔNp63 and cytokeratin 5 program, and subsequent Notch blockade promotes an alveolar cell fate. Persistent Notch signalling after injury led to parenchymal 'micro-honeycombing' (alveolar cysts), indicative of failed regeneration. Lungs from patients with fibrosis show analogous honeycomb cysts with evidence of hyperactive Notch signalling. Our findings indicate that distinct stem/progenitor cell pools repopulate injured tissue depending on the extent of the injury, and the outcomes of regeneration or fibrosis may depend in part on the dynamics of LNEP Notch signalling. | | | 25533958
 |
Taspase1-dependent TFIIA cleavage coordinates head morphogenesis by limiting Cdkn2a locus transcription.
Takeda, S; Sasagawa, S; Oyama, T; Searleman, AC; Westergard, TD; Cheng, EH; Hsieh, JJ
The Journal of clinical investigation
125
1203-14
2015
Show Abstract
Head morphogenesis requires complex signal relays to enable precisely coordinated proliferation, migration, and patterning. Here, we demonstrate that, during mouse head formation, taspase1-mediated (TASP1-mediated) cleavage of the general transcription factor TFIIA ensures proper coordination of rapid cell proliferation and morphogenesis by maintaining limited transcription of the negative cell cycle regulators p16Ink4a and p19Arf from the Cdkn2a locus. In mice, loss of TASP1 function led to catastrophic craniofacial malformations that were associated with inadequate cell proliferation. Compound deficiency of Cdkn2a, especially p16Ink4a deficiency, markedly reduced the craniofacial anomalies of TASP1-deficent mice. Furthermore, evaluation of mice expressing noncleavable TASP1 targets revealed that TFIIA is the principal TASP1 substrate that orchestrates craniofacial morphogenesis. ChIP analyses determined that noncleaved TFIIA accumulates at the p16Ink4a and p19Arf promoters to drive transcription of these negative regulators. In summary, our study elucidates a regulatory circuit comprising proteolysis, transcription, and proliferation that is pivotal for construction of the mammalian head. | | | 25664857
 |