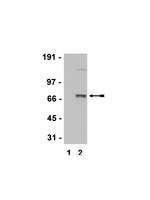
Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling.
Di Bartolo, V, et al.
J. Biol. Chem., 274: 6285-94 (1999)
1999
Show Abstract
Following T cell antigen receptor (TCR) engagement, the protein tyrosine kinase (PTK) ZAP-70 is rapidly phosphorylated on several tyrosine residues, presumably by two mechanisms: an autophosphorylation and a trans-phosphorylation by the Src-family PTK Lck. These events have been implicated in both positive and negative regulation of ZAP-70 activity and in coupling this PTK to downstream signaling pathways in T cells. We show here that Tyr315 and Tyr319 in the interdomain B of ZAP-70 are autophosphorylated in vitro and become phosphorylated in vivo upon TCR triggering. Moreover, by mutational analysis, we demonstrate that phosphorylation of Tyr319 is required for the positive regulation of ZAP-70 function. Indeed, overexpression in Jurkat cells and in a murine T cell hybridoma of a ZAP-70 mutant in which Tyr319 was replaced by phenylalanine (ZAP-70-Y319F) dramatically impaired anti-TCR-induced activation of the nuclear factor of activated T cells and interleukin-2 production, respectively. Surprisingly, an analogous mutation of Tyr315 had little or no effect. The inhibitory effect of ZAP-70-Y319F correlated with a substantial loss of its activation-induced tyrosine phosphorylation and up-regulation of catalytic activity, as well as with a decreased in vivo capacity to phosphorylate known ZAP-70 substrates, such as SLP-76 and LAT. Collectively, our data reveal the pivotal role of Tyr319 phosphorylation in the positive regulation of ZAP-70 and in TCR-mediated signaling. | 10037717
 |
Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation.
Williams, B L, et al.
EMBO J., 18: 1832-44 (1999)
1999
Show Abstract
Accumulating evidence indicates that the interdomain B regions of ZAP-70 and Syk play pivotal roles in the coupling of T-cell antigen receptor (TCR) stimulation to the activation of downstream signaling pathways. The interdomain B region of ZAP-70 contains at least three candidate sites of tyrosine phosphorylation. In this report, we identify Tyr319 as a functionally important phosphorylation site in the ZAP-70 interdomain B region. TCR crosslinkage triggered a rapid increase in the phosphorylation of Tyr319 in Jurkat T cells. Although mutation of Tyr319 to Phe had no effect on the tyrosine kinase activity of ZAP-70, the resulting ZAP(Y319-->F) mutant failed to reconstitute TCR-dependent Ca2+ mobilization, Ras activation, CD69 expression and NFAT-dependent transcription in ZAP-70-deficient Jurkat cells. These defects were correlated with reduced tyrosine phosphorylation of phospholipase C (PLC)-gamma1 and the LAT adapter protein in the ZAP(Y319-->F)-expressing cells. On the other hand, ZAP(Y319-->F)-expressing cells displayed normal increases in SLP-76 phosphorylation and ERK activation during TCR stimulation. Phosphorylation of Tyr319 promoted the association of ZAP-70 with the SH2 domains of two key signaling molecules, Lck and PLC-gamma1. These studies suggest that Tyr319 phosphorylation is required for the assembly of a ZAP-70-containing signaling complex that leads to the activation of the PLC-gamma1- and Ras-dependent signaling cascades in antigen-stimulated T cells. | 10202147
 |