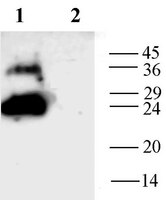
Altered expression of aquaporins in bullous keratopathy and Fuchs' dystrophy corneas.
M Cristina Kenney, Shari R Atilano, Nadia Zorapapel, Bret Holguin, Ronald N Gaster, Alexander V Ljubimov
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society
52
1341-50
2004
显示摘要
Corneas with edema-related diseases lose transparency, which causes significant vision loss. This study analyzed seven aquaporins (AQPs) in normal corneas, pseudophakic/aphakic bullous keratopathy (PBK/ABK) corneas, Fuchs' dystrophy corneas, keratoconus corneas, post-cataract surgery (PCS) corneas, and normal organ-cultured corneas. RNA levels for AQP1, AQP4, and beta2-microglobulin were measured by RT-PCR. AQP1 antibody localized to stromal cells of all corneas. PBK/ABK and Fuchs' dystrophy corneas had decreased endothelial cell staining compared with normal. AQP1 mRNA was found in whole corneas and cultured stromal fibroblasts but not in isolated epithelial cells. AQP3 staining was found in basal epithelial cells of the normal, Fuchs' dystrophy, and keratoconus corneas but throughout the entire epithelium of PBK/ABK corneas. AQP4 antibody localized to endothelial cells of all corneas and in stromal cells of PBK/ABK corneas. AQP4 mRNA was identified in whole human corneas. AQP5 was found in epithelial cells of all corneas. AQP0, AQP2, and AQP9 were not found in any corneas. Normal AQP distributions were found in PCS and organ-cultured corneas, although they showed signs of swelling. Our study demonstrates that AQP abnormalities are found in PBK/ABK corneas (decreased AQP1, increased AQP3 and AQP4) and Fuchs' dystrophy corneas (decreased AQP1). Although both have vision-disrupting corneal edema, the mechanisms of fluid accumulation may be different in each disease. | 15385580
 |
Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat.
King, L S, et al.
Am. J. Physiol., 273: C1541-8 (1997)
1997
显示摘要
Developmental expression of aquaporin water transport proteins is not well understood in respiratory tract or secretory glands; here we define aquaporin protein ontogeny in rat. Expression of aquaporin-3 (AQP3), AQP4, and AQP5 proteins occurs within 2 wk after birth, whereas AQP1 first appears before birth. In most tissues, aquaporin protein expression increases progressively, although transient high-level expression is noted in distal lung (AQP4 at postnatal day +2) and trachea (AQP5 at postnatal day +21 and AQP3 at postnatal day +42). In mature animals, AQP5 is abundant in distal lung and salivary glands, AQP3 and AQP4 are present in trachea, and AQP1 is present in all of these tissues except salivary glands. Surprisingly, all four aquaporin proteins are highly abundant in nasopharynx. Unlike AQP1, corticosteroids did not induce expression of AQP3, AQP4, or AQP5 in lung. Our results seemingly implicate aquaporins in proximal airway humidification, glandular secretion, and perinatal clearance of fluid from distal airways. However, the studies underscore a need for detailed immunohistochemical characterizations and definitive functional studies. | 9374639
 |
Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat.
Nielsen, S, et al.
Am. J. Physiol., 273: C1549-61 (1997)
1997
显示摘要
The molecular pathways for fluid transport in pulmonary, oral, and nasal tissues are still unresolved. Here we use immunocytochemistry and immunoelectron microscopy to define the sites of expression of four aquaporins in the respiratory tract and glandular epithelia, where they reside in distinct, nonoverlapping sites. Aquaporin-1 (AQP1) is present in apical and basolateral membranes of bronchial, tracheal, and nasopharyngeal vascular endothelium and fibroblasts. AQP5 is localized to the apical plasma membrane of type I pneumocytes and the apical plasma membranes of secretory epithelium in upper airway and salivary glands. In contrast, AQP3 is present in basal cells of tracheal and nasopharyngeal epithelium and is abundant in basolateral membranes of surface epithelial cells of nasal conchus. AQP4 resides in basolateral membranes of columnar cells of bronchial, tracheal, and nasopharyngeal epithelium; in nasal conchus AQP4 is restricted to basolateral membranes of a subset of intra- and subepithelial glands. These sites of expression suggest that transalveolar water movement, modulation of airway surface liquid, air humidification, and generation of nasopharyngeal secretions involve a coordinated network of aquaporin water channels. | 9374640
 |
Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family.
Preston, G M and Agre, P
Proc. Natl. Acad. Sci. U.S.A., 88: 11110-4 (1991)
1991
显示摘要
CHIP28 is a 28-kDa integral membrane protein with similarities to membrane channels and is found in erythrocytes and renal tubules. A cDNA for CHIP28 was isolated from human fetal liver cDNA template by a three-step polymerase chain reaction (PCR) cloning strategy, starting with degenerate oligonucleotide primers corresponding to the N-terminal amino acid sequence determined from purified CHIP28 protein. Using the third-step PCR product as a probe, we isolated a recombinant from a human bone marrow cDNA library. The combined sequence of the PCR products and bone marrow cDNA contains 38 base pairs of 5' untranslated nucleotide sequence, an 807-bp open reading frame, and approximately 2 kilobases of 3' untranslated sequence containing a polyadenylation signal. This corresponds to the 3.1-kilobase transcript identified by RNA blot-hybridization analysis. Authenticity of the deduced amino acid sequence of the CHIP28 protein C terminus was confirmed by expression and immunoblotting. Analysis of the deduced amino acid sequence suggests that CHIP28 protein contains six bilayer-spanning domains, two exofacial potential N-glycosylation sites, and intracellular N and C termini. Search of the DNA sequence data base revealed a strong homology with the major intrinsic protein of bovine lens, which is the prototype of an ancient but recently recognized family of membrane channels. These proteins are believed to form channels permeable to water and possibly other small molecules. CHIP28 shares homology with all known members of this channel family, and it is speculated that CHIP28 has a similar function. | 1722319
 |