A Distinct Perisynaptic Glial Cell Type Forms Tripartite Neuromuscular Synapses in the Drosophila Adult.
Strauss, AL; Kawasaki, F; Ordway, RW
PloS one
10
e0129957
2015
Show Abstract
Previous studies of Drosophila flight muscle neuromuscular synapses have revealed their tripartite architecture and established an attractive experimental model for genetic analysis of glial function in synaptic transmission. Here we extend these findings by defining a new Drosophila glial cell type, designated peripheral perisynaptic glia (PPG), which resides in the periphery and interacts specifically with fine motor axon branches forming neuromuscular synapses. Identification and specific labeling of PPG was achieved through cell type-specific RNAi-mediated knockdown (KD) of a glial marker, Glutamine Synthetase 2 (GS2). In addition, comparison among different Drosophila neuromuscular synapse models from adult and larval developmental stages indicated the presence of tripartite synapses on several different muscle types in the adult. In contrast, PPG appear to be absent from larval body wall neuromuscular synapses, which do not exhibit a tripartite architecture but rather are imbedded in the muscle plasma membrane. Evolutionary conservation of tripartite synapse architecture and peripheral perisynaptic glia in vertebrates and Drosophila suggests ancient and conserved roles for glia-synapse interactions in synaptic transmission. | | | 26053860
 |
Tfap2a and 2b act downstream of Ptf1a to promote amacrine cell differentiation during retinogenesis.
Jin, K; Jiang, H; Xiao, D; Zou, M; Zhu, J; Xiang, M
Molecular brain
8
28
2015
Show Abstract
Retinogenesis is a precisely controlled developmental process during which different types of neurons and glial cells are generated under the influence of intrinsic and extrinsic factors. Three transcription factors, Foxn4, RORβ1 and their downstream effector Ptf1a, have been shown to be indispensable intrinsic regulators for the differentiation of amacrine and horizontal cells. At present, however, it is unclear how Ptf1a specifies these two cell fates from competent retinal precursors. Here, through combined bioinformatic, molecular and genetic approaches in mouse retinas, we identify the Tfap2a and Tfap2b transcription factors as two major downstream effectors of Ptf1a. RNA-seq and immunolabeling analyses show that the expression of Tfap2a and 2b transcripts and proteins is dramatically downregulated in the Ptf1a null mutant retina. Their overexpression is capable of promoting the differentiation of glycinergic and GABAergic amacrine cells at the expense of photoreceptors much as misexpressed Ptf1a is, whereas their simultaneous knockdown has the opposite effect. Given the demonstrated requirement for Tfap2a and 2b in horizontal cell differentiation, our study thus defines a Foxn4/RORβ1-Ptf1a-Tfap2a/2b transcriptional regulatory cascade that underlies the competence, specification and differentiation of amacrine and horizontal cells during retinal development. | | | 25966682
 |
Inner retinal change in a novel rd1-FTL mouse model of retinal degeneration.
Greferath, U; Anderson, EE; Jobling, AI; Vessey, KA; Martinez, G; de Iongh, RU; Kalloniatis, M; Fletcher, EL
Frontiers in cellular neuroscience
9
293
2015
Show Abstract
While photoreceptor loss is the most devastating result of inherited retinal degenerations such as retinitis pigmentosa, inner retinal neurons also undergo significant alteration. Detailing these changes has become important as many vision restorative therapies target the remaining neurons. In this study, the rd1-Fos-Tau-LacZ (rd1-FTL) mouse model was used to explore inner retinal change at a late stage of retinal degeneration, after the loss of photoreceptor nuclei. The rd1-FTL model carries a mutation in the phosphodiesterase gene, Pde6b, and an axonally targeted transgenic beta galactosidase reporter system under the control of the c-fos promoter. Retinae of transgenic rd1-FTL mice and control FTL animals aged 2-12 months were processed for indirect fluorescence immunocytochemistry. At 2 months of age, a time when the majority of photoreceptor nuclei are lost, there was negligible c-fos reporter (FTL) expression, however, from 4 months, reporter expression was observed to increase within subpopulations of amacrine and ganglion cells within the central retina. These areas of inner retinal FTL expression coincided with regions that contained aberrant Müller cells. Specifically, these cells exhibited reduced glutamine synthetase and Kir4.1 immunolabelling, whilst showing evidence of proliferative gliosis (increased cyclinD1 and glial fibrillary acidic protein expression). These changes were limited to distinct regions where cone photoreceptor terminals were absent. Overall, these results highlight that distinct areas of the rd1-FTL central retina undergo significant glial alterations after cone photoreceptor loss. These areas coincide with up-regulation of the c-fos reporter in the inner retina, which may represent a change in neuronal function/plasticity. The rd1-FTL mouse is a useful model system to probe changes that occur in the inner retina at later stages of retinal degeneration. | | | 26283925
 |
Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration.
Scholz, R; Caramoy, A; Bhuckory, MB; Rashid, K; Chen, M; Xu, H; Grimm, C; Langmann, T
Journal of neuroinflammation
12
201
2015
Show Abstract
Reactive microglia are commonly seen in retinal degenerative diseases, and neurotoxic microglia responses can contribute to photoreceptor cell death. We and others have previously shown that translocator protein (18 kDa) (TSPO) is highly induced in retinal degenerations and that the selective TSPO ligand XBD173 (AC-5216, emapunil) exerts strong anti-inflammatory effects on microglia in vitro and ex vivo. Here, we investigated whether targeting TSPO with XBD173 has immuno-modulatory and neuroprotective functions in two mouse models of acute retinal degeneration using bright white light exposure.BALB/cJ and Cx3cr1(GFP/+) mice received intraperitoneal injections of 10 mg/kg XBD173 or vehicle for five consecutive days, starting 1 day prior to exposure to either 15,000 lux white light for 1 h or 50,000 lux focal light for 10 min, respectively. The effects of XBD173 treatment on microglia and Müller cell reactivity were analyzed by immuno-stainings of retinal sections and flat mounts, fluorescence-activated cell sorting (FACS) analysis, and mRNA expression of microglia markers using quantitative real-time PCR (qRT-PCR). Optical coherence tomography (OCT), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stainings, and morphometric analyses were used to quantify the extent of retinal degeneration and photoreceptor apoptosis.Four days after the mice were challenged with bright white light, a large number of amoeboid-shaped alerted microglia appeared in the degenerating outer retina, which was nearly completely prevented by treatment with XBD173. This treatment also down-regulated the expression of TSPO protein in microglia but did not change the TSPO levels in the retinal pigment epithelium (RPE). RT-PCR analysis showed that the microglia/macrophage markers Cd68 and activated microglia/macrophage whey acidic protein (Amwap) as well as the pro-inflammatory genes Ccl2 and Il6 were reduced after XBD173 treatment. Light-induced degeneration of the outer retina was nearly fully blocked by XBD173 treatment. We further confirmed these findings in an independent mouse model of focal light damage. Retinas of animals receiving XBD173 therapy displayed significantly more ramified non-reactive microglia and more viable arrestin-positive cone photoreceptors than vehicle controls.Targeting TSPO with XBD173 effectively counter-regulates microgliosis and ameliorates light-induced retinal damage, highlighting a new pharmacological concept for the treatment of retinal degenerations. | | | 26527153
 |
Induction of ectopic retina-like tissue by transgenic expression of neurogenin.
Yan, RT; He, L; Zhan, W; Wang, SZ
PloS one
10
e0116171
2015
Show Abstract
Degeneration of retinal neurons is an underlying cause of several major types of blinding diseases, and effective therapies remain to be developed. The suppositive strategy of repopulating a degenerative retina with new cells generated onsite faces serious challenges, because the mammalian retina seems to lack the ability to regenerate itself or replace its lost neurons. We investigated the possibility of using a transcriptional factor with proneural activities to reprogram ocular tissue with regenerative capability to give rise to retinal cells. Transgenic mice were generated with DNA constructs that targeted the expression in the retinal pigment epithelium of proneural gene neurogenin1 from the promoter of Bestrophin1, or neurogenin3 from RPE65 promoter. Here we report the presence of ectopic retina-like tissue in some of the transgenic mice, young and aged. The ectopic retina-like tissue contained cells positive for photoreceptor proteins Crx, recoverin, red opsin, and rhodopsin, and cells positive for proteins that label other types of retinal neurons, including AP2α and Pax6 for amacrine cells, Otx2 for bipolar cells, and Brn3A for ganglion cells. The retina-like tissue often co-existed with darkly pigmented tissue positive for RPE proteins: cytokeratin 18, Otx2, and RPE65. The ectopic retina-like tissue was detected in the subretinal space, including two retinae co-existing in the same eye, and/or in the optic nerve or in the vicinity of the optic nerve head. On rare occasions, it was detected in the choroid and in the vicinity of the ciliary body. The presence of ectopic retina-like tissue in the transgenic mouse supports the possibility of inducing retinal regeneration in the mammalian eyes through gene-directed reprograming. | | | 25635399
 |
Midkine-a protein localization in the developing and adult retina of the zebrafish and its function during photoreceptor regeneration.
Gramage, E; D'Cruz, T; Taylor, S; Thummel, R; Hitchcock, PF
PloS one
10
e0121789
2015
Show Abstract
Midkine is a heparin binding growth factor with important functions in neuronal development and survival, but little is known about its function in the retina. Previous studies show that in the developing zebrafish, Midkine-a (Mdka) regulates cell cycle kinetics in retinal progenitors, and following injury to the adult zebrafish retina, mdka is strongly upregulated in Müller glia and the injury-induced photoreceptor progenitors. Here we provide the first data describing Mdka protein localization during different stages of retinal development and during the regeneration of photoreceptors in adults. We also experimentally test the role of Mdka during photoreceptor regeneration. The immuno-localization of Mdka reflects the complex spatiotemporal pattern of gene expression and also reveals the apparent secretion and extracellular trafficking of this protein. During embryonic retinal development the Mdka antibodies label all mitotically active cells, but at the onset of neuronal differentiation, immunostaining is also localized to the nascent inner plexiform layer. Starting at five days post fertilization through the juvenile stage, Mdka immunostaining labels the cytoplasm of horizontal cells and the overlying somata of rod photoreceptors. Double immunolabeling shows that in adult horizontal cells, Mdka co-localizes with markers of the Golgi complex. Together, these data are interpreted to show that Mdka is synthesized in horizontal cells and secreted into the outer nuclear layer. In adults, Mdka is also present in the end feet of Müller glia. Similar to mdka gene expression, Mdka in horizontal cells is regulated by circadian rhythms. After the light-induced death of photoreceptors, Mdka immuonolabeling is localized to Müller glia, the intrinsic stem cells of the zebrafish retina, and proliferating photoreceptor progenitors. Knockdown of Mdka during photoreceptor regeneration results in less proliferation and diminished regeneration of rod photoreceptors. These data suggest that during photoreceptor regeneration Mdka regulates aspects of injury-induced cell proliferation. | | | 25803551
 |
Expression of leukemia inhibitory factor in Müller glia cells is regulated by a redox-dependent mRNA stability mechanism.
Agca, C; Boldt, K; Gubler, A; Meneau, I; Corpet, A; Samardzija, M; Stucki, M; Ueffing, M; Grimm, C
BMC biology
13
30
2015
Show Abstract
Photoreceptor degeneration is a main hallmark of many blinding diseases making protection of photoreceptors crucial to prevent vision loss. Thus, regulation of endogenous neuroprotective factors may be key for cell survival and attenuation of disease progression. Important neuroprotective factors in the retina include H2O2 generated by injured photoreceptors, and leukemia inhibitory factor (LIF) expressed in Müller glia cells in response to photoreceptor damage.We present evidence that H2O2 connects to the LIF response by inducing stabilization of Lif transcripts in Müller cells. This process was independent of active gene transcription and p38 MAPK, but relied on AU-rich elements (AREs), which we identified within the highly conserved Lif 3'UTR. Affinity purification combined with quantitative mass spectrometry identified several proteins that bound to these AREs. Among those, interleukin enhancer binding factor 3 (ILF3) was confirmed to participate in the redox-dependent Lif mRNA stabilization. Additionally we show that KH-type splicing regulatory protein (KHSRP) was crucial for maintaining basal Lif expression levels in non-stressed Müller cells.Our results suggest that H2O2-induced redox signaling increases Lif transcript levels through ILF3 mediated mRNA stabilization. Generation of H2O2 by injured photoreceptors may thus enhance stability of Lif mRNA and therefore augment neuroprotective LIF signaling during degenerative conditions in vivo. | | | 25907681
 |
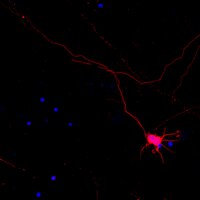
SOX2 reprograms resident astrocytes into neural progenitors in the adult brain.
Niu, W; Zang, T; Smith, DK; Vue, TY; Zou, Y; Bachoo, R; Johnson, JE; Zhang, CL
Stem cell reports
4
780-94
2015
Show Abstract
Glial cells can be in vivo reprogrammed into functional neurons in the adult CNS; however, the process by which this reprogramming occurs is unclear. Here, we show that a distinct cellular sequence is involved in SOX2-driven in situ conversion of adult astrocytes to neurons. This includes ASCL1(+) neural progenitors and DCX(+) adult neuroblasts (iANBs) as intermediates. Importantly, ASCL1 is required, but not sufficient, for the robust generation of iANBs in the adult striatum. These progenitor-derived iANBs predominantly give rise to calretinin(+) interneurons when supplied with neurotrophic factors or the small-molecule valproic acid. Patch-clamp recordings from the induced neurons reveal subtype heterogeneity, though all are functionally mature, fire repetitive action potentials, and receive synaptic inputs. Together, these results show that SOX2-mediated in vivo reprogramming of astrocytes to neurons passes through proliferative intermediate progenitors, which may be exploited for regenerative medicine. | | | 25921813
 |
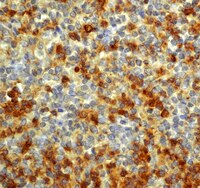
Diagnostic utility and limitations of glutamine synthetase and serum amyloid-associated protein immunohistochemistry in the distinction of focal nodular hyperplasia and inflammatory hepatocellular adenoma.
Joseph, NM; Ferrell, LD; Jain, D; Torbenson, MS; Wu, TT; Yeh, MM; Kakar, S
Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc
27
62-72
2014
Show Abstract
Inflammatory hepatocellular adenoma can show overlapping histological features with focal nodular hyperplasia, including inflammation, fibrous stroma, and ductular reaction. Expression of serum amyloid-associated protein in inflammatory hepatocellular adenoma and map-like pattern of glutamine synthetase in focal nodular hyperplasia can be helpful in this distinction, but the pitfalls and limitations of these markers have not been established. Morphology and immunohistochemistry were analyzed in 54 inflammatory hepatocellular adenomas, 40 focal nodular hyperplasia, and 3 indeterminate lesions. Morphological analysis demonstrated that nodularity, fibrous stroma, dystrophic blood vessels, and ductular reaction were more common in focal nodular hyperplasia, while telangiectasia, hemorrhage, and steatosis were more common in inflammatory hepatocellular adenoma, but there was frequent overlap of morphological features. The majority of inflammatory hepatocellular adenomas demonstrated perivascular and/or patchy glutamine synthetase staining (73.6%), while the remaining cases had diffuse (7.5%), negative (3.8%), or patchy pattern of staining (15%) that showed subtle differences from the classic map-like staining pattern and was designated as pseudo map-like staining. Positive staining for serum amyloid-associated protein was seen in the majority of inflammatory hepatocellular adenomas (92.6%) and in the minority of focal nodular hyperplasia (17.5%). The glutamine synthetase staining pattern was map-like in 90% of focal nodular hyperplasia cases, with the remaining 10% of cases showing pseudo map-like staining. Three cases were labeled as indeterminate and showed focal nodular hyperplasia-like morphology but lacked map-like glutamine synthetase staining pattern; these cases demonstrated a patchy pseudo map-like glutamine synthetase pattern along with the expression of serum amyloid-associated protein. Our results highlight the diagnostic errors that can be caused by variant patterns of staining with glutamine synthetase and serum amyloid-associated protein in inflammatory hepatocellular adenoma and focal nodular hyperplasia. | | | 23807780
 |
Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain.
Sosunov, AA; Wu, X; Tsankova, NM; Guilfoyle, E; McKhann, GM; Goldman, JE
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
2285-98
2014
Show Abstract
To examine the diversity of astrocytes in the human brain, we immunostained surgical specimens of temporal cortex and hippocampus and autopsy brains for CD44, a plasma membrane protein and extracellular matrix receptor. CD44 antibodies outline the details of astrocyte morphology to a degree not possible with glial fibrillary acidic protein (GFAP) antibodies. CD44+ astrocytes could be subdivided into two groups. First, CD44+ astrocytes with long processes were consistently found in the subpial area ("interlaminar" astrocytes), the deep isocortical layers, and the hippocampus. Many of these processes ended on blood vessels. Some were also found adjacent to large blood vessels, from which they extended long processes. We observed these CD44+, long-process astrocytes in every brain we examined, from fetal to adult. These astrocytes generally displayed high immunostaining for GFAP, S100β, and CD44, but low immunostaining for glutamine synthetase, excitatory amino-acid transporter 1 (EAAT1), and EAAT2. Aquaporin 4 (AQP4) appeared distributed all over the cell bodies and processes of the CD44+ astrocytes, while, in contrast, AQP4 localized to perivascular end feet in the CD44- protoplasmic astrocytes. Second, there were CD44+ astrocytes without long processes in the cortex. These were not present during gestation or at birth, and in adult brains varied substantially in number, shape, and immunohistochemical phenotype. Many of these displayed a "mixed" morphological and immunocytochemical phenotype between protoplasmic and fibrous astrocytes. We conclude that the diversity of astrocyte populations in the isocortex and archicortex in the human brain reflects both intrinsic and acquired phenotypes, the latter perhaps representing a shift from CD44- "protoplasmic" to CD44+ "fibrous"-like astrocytes. | | | 24501367
 |