Loss of Drosophila Ataxin-7, a SAGA subunit, reduces H2B ubiquitination and leads to neural and retinal degeneration.
Mohan, RD; Dialynas, G; Weake, VM; Liu, J; Martin-Brown, S; Florens, L; Washburn, MP; Workman, JL; Abmayr, SM
Genes & development
28
259-72
2014
Show Abstract
The Spt-Ada-Gcn5-acetyltransferase (SAGA) chromatin-modifying complex possesses acetyltransferase and deubiquitinase activities. Within this modular complex, Ataxin-7 anchors the deubiquitinase activity to the larger complex. Here we identified and characterized Drosophila Ataxin-7 and found that reduction of Ataxin-7 protein results in loss of components from the SAGA complex. In contrast to yeast, where loss of Ataxin-7 inactivates the deubiquitinase and results in increased H2B ubiquitination, loss of Ataxin-7 results in decreased H2B ubiquitination and H3K9 acetylation without affecting other histone marks. Interestingly, the effect on ubiquitination was conserved in human cells, suggesting a novel mechanism regulating histone deubiquitination in higher organisms. Consistent with this mechanism in vivo, we found that a recombinant deubiquitinase module is active in the absence of Ataxin-7 in vitro. When we examined the consequences of reduced Ataxin-7 in vivo, we found that flies exhibited pronounced neural and retinal degeneration, impaired movement, and early lethality. | | 24493646
 |
The histone H2A deubiquitinase Usp16 regulates embryonic stem cell gene expression and lineage commitment.
Yang, W; Lee, YH; Jones, AE; Woolnough, JL; Zhou, D; Dai, Q; Wu, Q; Giles, KE; Townes, TM; Wang, H
Nature communications
5
3818
2014
Show Abstract
Polycomb Repressive Complex 1 and histone H2A ubiquitination (ubH2A) contribute to embryonic stem cell (ESC) pluripotency by repressing lineage-specific gene expression. However, whether active deubiquitination co-regulates ubH2A levels in ESCs and during differentiation is not known. Here we report that Usp16, a histone H2A deubiquitinase, regulates H2A deubiquitination and gene expression in ESCs, and importantly, is required for ESC differentiation. Usp16 knockout is embryonic lethal in mice, but does not affect ESC viability or identity. Usp16 binds to the promoter regions of a large number of genes in ESCs, and Usp16 binding is inversely correlated with ubH2A levels, and positively correlates with gene expression levels. Intriguingly, Usp16(-/-) ESCs fail to differentiate due to ubH2A-mediated repression of lineage-specific genes. Finally, Usp16, but not a catalytically inactive mutant, rescues the differentiation defects of Usp16(-/-) ESCs. Therefore, this study identifies Usp16 and H2A deubiquitination as critical regulators of ESC gene expression and differentiation. | | 24784029
 |
Histone H2B ubiquitin ligase RNF20 is required for MLL-rearranged leukemia.
Wang, E; Kawaoka, S; Yu, M; Shi, J; Ni, T; Yang, W; Zhu, J; Roeder, RG; Vakoc, CR
Proceedings of the National Academy of Sciences of the United States of America
110
3901-6
2013
Show Abstract
Mixed-lineage leukemia (MLL) fusions are potent oncogenes that initiate aggressive forms of acute leukemia. As aberrant transcriptional regulators, MLL-fusion proteins alter gene expression in hematopoietic cells through interactions with the histone H3 lysine 79 (H3K79) methyltransferase DOT1L. Notably, interference with MLL-fusion cofactors like DOT1L is an emerging therapeutic strategy in this disease. Here, we identify the histone H2B E3 ubiquitin ligase ring finger protein 20 (RNF20) as an additional chromatin regulator that is necessary for MLL-fusion-mediated leukemogenesis. Suppressing the expression of Rnf20 in diverse models of MLL-rearranged leukemia leads to inhibition of cell proliferation, under tissue culture conditions as well as in vivo. Rnf20 knockdown leads to reduced expression of MLL-fusion target genes, effects resembling Dot1l inhibition. Using ChIP-seq, we found that H2B ubiquitination is enriched in the body of MLL-fusion target genes, correlating with sites of H3K79 methylation and transcription elongation. Furthermore, Rnf20 is required to maintain local levels of H3K79 methylation by Dot1l at Hoxa9 and Meis1. These findings support a model whereby cotranscriptional recruitment of Rnf20 at MLL-fusion target genes leads to amplification of Dot1l-mediated H3K79 methylation, thereby rendering leukemia cells dependent on Rnf20 to maintain their oncogenic transcriptional program. | | 23412334
 |
The ZFP-1(AF10)/DOT-1 complex opposes H2B ubiquitination to reduce Pol II transcription.
Cecere, G; Hoersch, S; Jensen, MB; Dixit, S; Grishok, A
Molecular cell
50
894-907
2013
Show Abstract
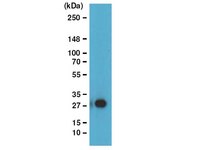
The inhibition of transcriptional elongation plays an important role in gene regulation in metazoans, including C. elegans. Here, we combine genomic and biochemical approaches to dissect a role of ZFP-1, the C. elegans AF10 homolog, in transcriptional control. We show that ZFP-1 and its interacting partner DOT-1.1 have a global role in negatively modulating the level of polymerase II (Pol II) transcription on essential widely expressed genes. Moreover, the ZFP-1/DOT-1.1 complex contributes to progressive Pol II pausing on essential genes during development and to rapid Pol II pausing during stress response. The slowing down of Pol II transcription by ZFP-1/DOT-1.1 is associated with an increase in H3K79 methylation and a decrease in H2B monoubiquitination, which promotes transcription. We propose a model wherein the recruitment of ZFP-1/DOT-1.1 and deposition of H3K79 methylation at highly expressed genes initiates a negative feedback mechanism for the modulation of their expression. | Western Blotting | 23806335
 |
ASH2L regulates ubiquitylation signaling to MLL: trans-regulation of H3 K4 methylation in higher eukaryotes.
Wu, L; Lee, SY; Zhou, B; Nguyen, UT; Muir, TW; Tan, S; Dou, Y
Molecular cell
49
1108-20
2013
Show Abstract
Crosstalk between H2B ubiquitylation (H2Bub) and H3 K4 methylation plays important roles in coordinating functions of diverse cofactors during transcription activation. The underlying mechanism for this trans-tail signaling pathway is poorly defined in higher eukaryotes. Here, we show the following: (1) ASH2L in the MLL complex is essential for H2Bub-dependent H3 K4 methylation. Deleting or mutating K99 of the N-terminal winged helix (WH) motif in ASH2L abrogates H2Bub-dependent regulation. (2) Crosstalk can occur in trans and does not require ubiquitin to be on nucleosomes or histones to exert regulatory effects. (3) trans-regulation by ubiquitin promotes MLL activity for all three methylation states. (4) MLL3, an MLL homolog, does not respond to H2Bub, highlighting regulatory specificity for MLL family histone methyltransferases. Altogether, our results potentially expand the classic histone crosstalk to nonhistone proteins, which broadens the scope of chromatin regulation by ubiquitylation signaling. | | 23453805
 |
USP49 deubiquitinates histone H2B and regulates cotranscriptional pre-mRNA splicing.
Zhang, Z; Jones, A; Joo, HY; Zhou, D; Cao, Y; Chen, S; Erdjument-Bromage, H; Renfrow, M; He, H; Tempst, P; Townes, TM; Giles, KE; Ma, L; Wang, H
Genes & development
27
1581-95
2013
Show Abstract
Post-translational histone modifications play important roles in regulating chromatin structure and function. Histone H2B ubiquitination and deubiquitination have been implicated in transcriptional regulation, but the function of H2B deubiquitination is not well defined, particularly in higher eukaryotes. Here we report the purification of ubiquitin-specific peptidase 49 (USP49) as a histone H2B-specific deubiquitinase and demonstrate that H2B deubiquitination by USP49 is required for efficient cotranscriptional splicing of a large set of exons. USP49 forms a complex with RuvB-like1 (RVB1) and SUG1 and specifically deubiquitinates histone H2B in vitro and in vivo. USP49 knockdown results in small changes in gene expression but affects the abundance of greater than 9000 isoforms. Exons down-regulated in USP49 knockdown cells show both elevated levels of alternative splicing and a general decrease in splicing efficiency. Importantly, USP49 is relatively enriched at this set of exons. USP49 knockdown increased H2B ubiquitination (uH2B) levels at these exons as well as upstream 3' and downstream 5' intronic splicing elements. Change in H2B ubiquitination level, as modulated by USP49, regulates U1A and U2B association with chromatin and binding to nascent pre-mRNA. Although H3 levels are relatively stable after USP49 depletion, H2B levels at these exons are dramatically increased, suggesting that uH2B may enhance nucleosome stability. Therefore, this study identifies USP49 as a histone H2B-specific deubiquitinase and uncovers a critical role for H2B deubiquitination in cotranscriptional pre-mRNA processing events. | | 23824326
 |
Decreased histone H2B monoubiquitination in malignant gastric carcinoma.
Wang, ZJ; Yang, JL; Wang, YP; Lou, JY; Chen, J; Liu, C; Guo, LD
World journal of gastroenterology
19
8099-107
2013
Show Abstract
To investigate H2B monoubiquitination (uH2B) and H3K4 di- and tri-methylation (H3K4-2me, H3K4-3me) levels and their clinical significance in gastric cancer (GC).Immunohistochemistry (IGC) was used to detect the differential levels of uH2B, H3K4-2me and H3K4-3me modifications in GC specimens from chemo/radiotherapy-naïve patients who underwent potentially curative surgical resection (n = 159) and in a random sampling of non-tumor gastric epithelium specimens (normal controls, n = 20). The immunohistochemistry (IHC)-detected modifications were classified as negative, low-level, or high-level using a dual-rated (staining intensity and percentage of positively-stained cells) semi-quantitative method. The relationships between uH2B modification levels and clinicopathological parameters of GC were assessed by a Wilcoxon rank sum test (pairwise comparisons) and the Kruskal-Wallis H test (multiple comparisons). The correlation between uH2B modification and survival was estimated by Kaplan-Meier analysis, and the role of uH2B as an independent prognostic factor for survival was assessed by multivariate Cox regression analysis.The presence and level of H3K4-2me and H3K4-3me IHC staining was similar between the normal controls and GC specimens. In contrast, the level of uH2B was significantly lower in the malignant gastric tissues (vs normal control tissues) and decreased along with increases in dedifferentiation (well differentiated greater than moderately differentiated greater than poorly differentiated). The level of uH2B correlated with tumor differentiation (P less than 0.001), Lauren's diffuse- and intestinal-type classification (P less than 0.001), lymph node metastasis (P = 0.049) and tumor-node-metastasis stage (P = 0.005). Patients with uH2B+ staining had higher 5-year survival rates than patients with uH2B-staining (52.692 ± 2.452 vs 23.739 ± 5.207, P less than 0.001). The uH2B level was an independent prognostic factor for cancer-specific survival (95%CI: 0.237-0.677, P = 0.001).uH2B displays differential IHC staining patterns corresponding to progressive stages of GC. uH2B may contribute to tumorigenesis and could be a potential therapeutic target. | | 24307806
 |
The deubiquitylating enzyme USP44 counteracts the DNA double-strand break response mediated by the RNF8 and RNF168 ubiquitin ligases.
Mosbech, A; Lukas, C; Bekker-Jensen, S; Mailand, N
The Journal of biological chemistry
288
16579-87
2013
Show Abstract
Protein recruitment to DNA double-strand breaks (DSBs) relies on ubiquitylation of the surrounding chromatin by the RING finger ubiquitin ligases RNF8 and RNF168. Flux through this pathway is opposed by several deubiquitylating enzymes (DUBs), including OTUB1 and USP3. By analyzing the effect of individually overexpressing the majority of human DUBs on RNF8/RNF168-mediated 53BP1 retention at DSB sites, we found that USP44 and USP29 powerfully inhibited this response at the level of RNF168 accrual. Both USP44 and USP29 promoted efficient deubiquitylation of histone H2A, but unlike USP44, USP29 displayed nonspecific reactivity toward ubiquitylated substrates. Moreover, USP44 but not other H2A DUBs was recruited to RNF168-generated ubiquitylation products at DSB sites. Individual depletion of these DUBs only mildly enhanced accumulation of ubiquitin conjugates and 53BP1 at DSBs, suggesting considerable functional redundancy among cellular DUBs that restrict ubiquitin-dependent protein assembly at DSBs. Our findings implicate USP44 in negative regulation of the RNF8/RNF168 pathway and illustrate the usefulness of DUB overexpression screens for identification of antagonizers of ubiquitin-dependent cellular responses. | | 23615962
 |
Transcription factor Sp3 represses expression of p21CIP¹ via inhibition of productive elongation by RNA polymerase II.
Valin, A; Ouyang, J; Gill, G
Molecular and cellular biology
33
1582-93
2013
Show Abstract
Like that of many protein-coding genes, expression of the p21(CIP1) cell cycle inhibitor is regulated at the level of transcription elongation. While many transcriptional activators have been shown to stimulate elongation, the mechanisms by which promoter-specific repressors regulate pausing and elongation by RNA polymerase II (RNA PolII) are not well described. Here we report that the transcription factor Sp3 inhibits basal p21(CIP1) gene expression by promoter-bound RNA PolII. Knockdown of Sp3 led to increased p21(CIP1) mRNA levels and reduced occupancy of the negative elongation factor (NELF) at the p21(CIP1) promoter, although the level of binding of the positive transcription elongation factor b (P-TEFb) kinase was not increased. Sp3 depletion correlated with increased H3K36me3 and H2Bub1, two histone modifications associated with transcription elongation. Further, Sp3 was shown to promote the binding of protein phosphatase 1 (PP1) to the p21(CIP1) promoter, leading to reduced H3S10 phosphorylation, a finding consistent with Sp3-dependent regulation of the local balance between kinase and phosphatase activities. Analysis of other targets of Sp3-mediated repression suggests that, in addition to previously described SUMO modification-dependent chromatin-silencing mechanisms, inhibition of the transition of paused RNA PolII to productive elongation, described here for p21(CIP1), is a general mechanism by which transcription factor Sp3 fine-tunes gene expression. | | 23401853
 |
Putative molecular mechanism underlying sperm chromatin remodelling is regulated by reproductive hormones.
Gill-Sharma, MK; Choudhuri, J; Ansari, MA; D'Souza, S
Clinical epigenetics
4
23
2012
Show Abstract
The putative regulatory role of the male reproductive hormones in the molecular mechanism underlying chromatin condensation remains poorly understood. In the past decade, we developed two adult male rat models wherein functional deficits of testosterone or FSH, produced after treatments with 20 mg/Kg/d of cyproterone acetate (CPA) per os, for a period of 15 days or 3 mg/Kg/d of fluphenazine decanoate (FD) subcutaneously, for a period of 60 days, respectively, affected the rate of sperm chromatin decondensation in vitro. These rat models have been used in the current study in order to delineate the putative roles of testosterone and FSH in the molecular mechanism underlying remodelling of sperm chromatin.We report that deficits of both testosterone and FSH affected the turnover of polyubiquitylated histones and led to their accumulation in the testis. Functional deficits of testosterone reduced expression of MIWI, the 5-methyl cap binding RNA-binding protein (PIWIlike murine homologue of the Drosophila protein PIWI/P-element induced wimpy testis) containing a PAZ/Piwi-Argonaut-Zwille domain and levels of histone deacetylase1 (HDAC1), ubiquitin ligating enzyme (URE-B1/E3), 20S proteasome α1 concomitant with reduced expression of ubiquitin activating enzyme (ube1), conjugating enzyme (ube2d2), chromodomain Y like protein (cdyl), bromodomain testis specific protein (brdt), hdac6 (histone deacetylase6), androgen-dependent homeobox placentae embryonic protein (pem/RhoX5), histones h2b and th3 (testis-specific h3). Functional deficits of FSH reduced the expression of cdyl and brdt genes in the testis, affected turnover of ubiquitylated histones, stalled the physiological DNA repair mechanism and culminated in spermiation of DNA damaged sperm.We aver that deficits of both testosterone and FSH differentially affected the process of sperm chromatin remodelling through subtle changes in the 'chromatin condensation transcriptome and proteome', thereby stalling the replacement of 'dynamic' histones with 'inert' protamines, and altering the epigenetic state of condensed sperm chromatin. The inappropriately condensed chromatin affected the sperm chromatin cytoarchitecture, evident from subtle ultrastructural changes in the nuclei of immature caput epididymal sperm of CPA- or FD-treated rats, incubated in vitro with dithiothreitol. | Western Blotting | 23241214
 |