Bub1 autophosphorylation feeds back to regulate kinetochore docking and promote localized substrate phosphorylation.
Asghar, A; Lajeunesse, A; Dulla, K; Combes, G; Thebault, P; Nigg, EA; Elowe, S
6
2015
| | | 26399325
 |
The EBNA3 family of Epstein-Barr virus nuclear proteins associates with the USP46/USP12 deubiquitination complexes to regulate lymphoblastoid cell line growth.
Ohashi, M; Holthaus, AM; Calderwood, MA; Lai, CY; Krastins, B; Sarracino, D; Johannsen, E
PLoS pathogens
11
e1004822
2015
显示摘要
The Epstein-Barr virus (EBV) nuclear proteins EBNA3A, EBNA3B, and EBNA3C interact with the cell DNA binding protein RBPJ and regulate cell and viral genes. Repression of the CDKN2A tumor suppressor gene products p16(INK4A) and p14(ARF) by EBNA3A and EBNA3C is critical for EBV mediated transformation of resting B lymphocytes into immortalized lymphoblastoid cell lines (LCLs). To define the composition of endogenous EBNA3 protein complexes, we generated lymphoblastoid cell lines (LCLs) expressing flag-HA tagged EBNA3A, EBNA3B, or EBNA3C and used tandem affinity purification to isolate each EBNA3 complex. Our results demonstrated that each EBNA3 protein forms a distinct complex with RBPJ. Mass-spectrometry revealed that the EBNA3A and EBNA3B complexes also contained the deubquitylation complex consisting of WDR48, WDR20, and USP46 (or its paralog USP12) and that EBNA3C complexes contained WDR48. Immunoprecipitation confirmed that EBNA3A, EBNA3B, and EBNA3C association with the USP46 complex. Using chromatin immunoprecipitation, we demonstrate that WDR48 and USP46 are recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Mapping studies were consistent with WDR48 being the primary mediator of EBNA3 association with the DUB complex. By ChIP assay, WDR48 was recruited to the p14(ARF) promoter in an EBNA3C dependent manner. Importantly, WDR48 associated with EBNA3A and EBNA3C domains that are critical for LCL growth, suggesting a role for USP46/USP12 in EBV induced growth transformation. | | | 25855980
 |
Critical Function of γH2A in S-Phase.
Mejia-Ramirez, E; Limbo, O; Langerak, P; Russell, P
PLoS genetics
11
e1005517
2015
显示摘要
Phosphorylation of histone H2AX by ATM and ATR establishes a chromatin recruitment platform for DNA damage response proteins. Phospho-H2AX (γH2AX) has been most intensively studied in the context of DNA double-strand breaks caused by exogenous clastogens, but recent studies suggest that DNA replication stress also triggers formation of γH2A (ortholog of γH2AX) in Schizosaccharomyces pombe. Here, a focused genetic screen in fission yeast reveals that γH2A is critical when there are defects in Replication Factor C (RFC), which loads proliferating cell nuclear antigen (PCNA) clamp onto duplex DNA. Surprisingly Chk1, Cds1/Chk2 and the Rad9-Hus1-Rad1 checkpoint clamp, which are crucial for surviving many genotoxins, are fully dispensable in RFC-defective cells. Immunoblot analysis confirms that Rad9-Hus1-Rad1 is not required for formation of γH2A by Rad3/ATR in S-phase. Defects in DNA polymerase epsilon, which binds PCNA in the replisome, also create an acute need for γH2A. These requirements for γH2A were traced to its role in docking with Brc1, which is a 6-BRCT-domain protein that is structurally related to budding yeast Rtt107 and mammalian PTIP. Brc1, which localizes at stalled replication forks by binding γH2A, prevents aberrant formation of Replication Protein A (RPA) foci in RFC-impaired cells, suggesting that Brc1-coated chromatin stabilizes replisomes when PCNA or DNA polymerase availability limits DNA synthesis. | | | 26368543
 |
Mutation of histone H3 serine 86 disrupts GATA factor Ams2 expression and precise chromosome segregation in fission yeast.
Lim, KK; Ong, TY; Tan, YR; Yang, EG; Ren, B; Seah, KS; Yang, Z; Tan, TS; Dymock, BW; Chen, ES
2015
| | |
 |
The nucleosome acidic patch plays a critical role in RNF168-dependent ubiquitination of histone H2A.
Mattiroli, F; Uckelmann, M; Sahtoe, DD; van Dijk, WJ; Sixma, TK
2014
| | | 24518117
 |
Uncoupling transcription from covalent histone modification.
Zhang, H; Gao, L; Anandhakumar, J; Gross, DS
PLoS genetics
2014
| | | |
The histone H2A deubiquitinase Usp16 regulates embryonic stem cell gene expression and lineage commitment.
Yang, W; Lee, YH; Jones, AE; Woolnough, JL; Zhou, D; Dai, Q; Wu, Q; Giles, KE; Townes, TM; Wang, H
Nature communications
5
3818
2014
显示摘要
Polycomb Repressive Complex 1 and histone H2A ubiquitination (ubH2A) contribute to embryonic stem cell (ESC) pluripotency by repressing lineage-specific gene expression. However, whether active deubiquitination co-regulates ubH2A levels in ESCs and during differentiation is not known. Here we report that Usp16, a histone H2A deubiquitinase, regulates H2A deubiquitination and gene expression in ESCs, and importantly, is required for ESC differentiation. Usp16 knockout is embryonic lethal in mice, but does not affect ESC viability or identity. Usp16 binds to the promoter regions of a large number of genes in ESCs, and Usp16 binding is inversely correlated with ubH2A levels, and positively correlates with gene expression levels. Intriguingly, Usp16(-/-) ESCs fail to differentiate due to ubH2A-mediated repression of lineage-specific genes. Finally, Usp16, but not a catalytically inactive mutant, rescues the differentiation defects of Usp16(-/-) ESCs. Therefore, this study identifies Usp16 and H2A deubiquitination as critical regulators of ESC gene expression and differentiation. | | | 24784029
 |
Phosphorylation and arginine methylation mark histone H2A prior to deposition during Xenopus laevis development.
Wang, WL; Anderson, LC; Nicklay, JJ; Chen, H; Gamble, MJ; Shabanowitz, J; Hunt, DF; Shechter, D
2014
| | |
 |
Histone content increases in differentiating embryonic stem cells.
Karnavas, T; Pintonello, L; Agresti, A; Bianchi, ME
Frontiers in physiology
5
330
2014
显示摘要
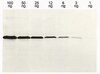
Mouse Embryonic Stem Cells (ESCs) are pluripotent mammalian cells derived from the Inner Cell Mass (ICM) of mouse blastocysts, which give rise to all three embryonic germ layers both in vivo and in vitro. Mouse ESCs have a distinct epigenetic landscape and a more decondensed chromatin compared to differentiated cells. Numerous studies have shown that distinct histone modifications in ESCs serve as hallmarks of pluripotency. However, so far it is still unknown whether the total histone content (as opposed to histone modifications) remains the same in cells of different developmental stage and differentiation capacity. In this work we show that total histone content differs between pluripotent and differentiated cells. In vitro spontaneous differentiation from ESCs to Embryoid Bodies (EBs) and directed differentiation toward neuronal and endodermal cells entails an increase in histone content. Primary MEFs also contain more histones than ESCs. We suggest that the difference in histone content is an additional hallmark of pluripotency, in addition to and besides histone modifications. | Western Blotting | | 25221520
 |
Coordinate nuclear targeting of the FANCD2 and FANCI proteins via a FANCD2 nuclear localization signal.
Boisvert, RA; Rego, MA; Azzinaro, PA; Mauro, M; Howlett, NG
PloS one
8
e81387
2013
显示摘要
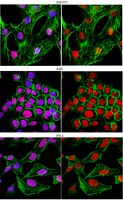
Fanconi anemia (FA) is a rare recessive disease, characterized by congenital defects, bone marrow failure, and increased cancer susceptibility. FA is caused by biallelic mutation of any one of sixteen genes. The protein products of these genes function cooperatively in the FA-BRCA pathway to repair DNA interstrand crosslinks (ICLs). A central step in the activation of this pathway is the monoubiquitination of the FANCD2 and FANCI proteins. Monoubiquitinated FANCD2 and FANCI localize to discrete chromatin regions where they function in ICL repair. Despite their critical role in ICL repair, very little is known about the structure, function, and regulation of the FANCD2 and FANCI proteins, or how they are targeted to the nucleus and chromatin. In this study, we describe the functional characterization of an amino-terminal FANCD2 nuclear localization signal (NLS). We demonstrate that the amino terminal 58 amino acids of FANCD2 can promote the nuclear expression of GFP and is necessary for the nuclear localization of FANCD2. Importantly, mutation of this FANCD2 NLS reveals that intact FANCD2 is required for the nuclear localization of a subset of FANCI. In addition, the NLS is necessary for the efficient monoubiquitination of FANCD2 and FANCI and, consequently, for their localization to chromatin. As a result, FANCD2 NLS mutants fail to rescue the ICL sensitivity of FA-D2 patient cells. Our studies yield important insight into the domain structure of the poorly characterized FANCD2 protein, and reveal a previously unknown mechanism for the coordinate nuclear import of a subset of FANCD2 and FANCI, a key early step in the cellular ICL response. | Western Blotting | Human | 24278431
 |