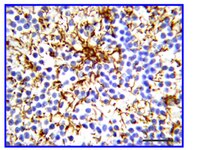
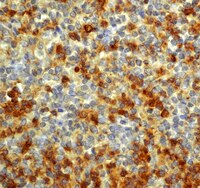
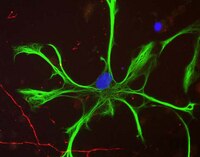
Dopamine D1 receptor activation regulates the expression of the estrogen synthesis gene aromatase B in radial glial cells.
Xing, L; McDonald, H; Da Fonte, DF; Gutierrez-Villagomez, JM; Trudeau, VL
Frontiers in neuroscience
9
310
2015
Show Abstract
Radial glial cells (RGCs) are abundant stem-like non-neuronal progenitors that are important for adult neurogenesis and brain repair, yet little is known about their regulation by neurotransmitters. Here we provide evidence for neuronal-glial interactions via a novel role for dopamine to stimulate RGC function. Goldfish were chosen as the model organism due to the abundance of RGCs and regenerative abilities of the adult central nervous system. A close anatomical relationship was observed between tyrosine hydroxylase-positive catecholaminergic cell bodies and axons and dopamine-D1 receptor expressing RGCs along the ventricular surface of telencephalon, a site of active neurogenesis. A primary cell culture model was established and immunofluorescence analysis indicates that in vitro RGCs from female goldfish retain their major characteristics in vivo, including expression of glial fibrillary acidic protein and brain lipid binding protein. The estrogen synthesis enzyme aromatase B is exclusively found in RGCs, but this is lost as cells differentiate to neurons and other glial types in adult teleost brain. Pharmacological experiments using the cultured RGCs established that specific activation of dopamine D1 receptors up-regulates aromatase B mRNA through a cyclic adenosine monophosphate-dependent molecular mechanism. These data indicate that dopamine enhances the steroidogenic function of this neuronal progenitor cell. | | | 26388722
 |
Effects of dextromethorphan and oxycodone on treatment of neuropathic pain in mice.
Yang, PP; Yeh, GC; Huang, EY; Law, PY; Loh, HH; Tao, PL
Journal of biomedical science
22
81
2015
Show Abstract
Neuropathic pain is a very troublesome and difficult pain to treat. Although opioids are the best analgesics for cancer and surgical pain in clinic, only oxycodone among opioids shows better efficacy to alleviate neuropathic pain. However, many side effects associated with the use of oxycodone render the continued use of it in neuropathic pain treatment undesirable. Hence, we explored whether dextromethorphan (DM, a known N-methyl-D-aspartate receptor antagonist with neuroprotective properties) could potentiate the anti-allodynic effect of oxycodone and underlying mechanisms regarding to glial cells (astrocytes and microglia) activation and proinflammatory cytokines release in a spinal nerve injury (SNL) mice model.Oxycodone produced a dose-dependent anti-allodynic effect. Co-administration of DM at a dose of 10 mg/kg (i.p.) (DM10) which had no anti-allodynic effect by itself enhanced the acute oxycodone (1 mg/kg, s.c.) effect. When the chronic anti-allodynic effects were examined, co-administration of DM10 also significantly enhanced the oxycodone effect at 3 mg/kg. Furthermore, oxycodone decreased SNL-induced activation of glial cells (astrocytes and microglia) and plasma levels of proinflammatory cytokines (IL-6, IL-1β and TNF-α). Co-administration of DM10 potentiated these effects of oxycodone.The combined use of DM with oxycodone may have therapeutic potential for decreasing the effective dose of oxycodone on the treatment of neuropathic pain. Attenuation of the glial activation and proinflammatory cytokines in the spinal cord may be important mechanisms for these effects of DM. | | | 26391752
 |
Buyang Huanwu decoction increases the expression of glutamate transporter-1 and glutamate synthetase in association with PACAP-38 following focal ischemia.
Ding, W; Yu, P; Liu, W; Zhou, L; Guan, LI; Lin, R
Biomedical reports
3
651-656
2015
Show Abstract
The neuroprotective role of Buyang Huanwu decoction (BYHWD) in focal ischemia is associated with decreasing glutamate concentration. However, the mechanisms are not fully understood. The present study aimed to explore whether glutamate transporter-1 (GLT-1) and glutamine synthetase (GS) participated in the decreased level of glutamate and whether pituitary adenylate cyclase-activating polypeptide-38 (PACAP-38) was involved in this process. BYHWD was found to significantly upregulate the expression of GLT-1 and GS in the hippocampal CA1 area compared to the ischemia group, with the difference on day 3 being most significant. BYHWD increased the level of PACAP-38, and PACAP-(6-38) (PACAP receptor antagonist) significantly attenuated the effect of BYHWD on GLT-1 and GS, suggesting that PACAP-38 was involved in the upregulation of GLT-1 and GS induced by BYHWD. In addition, as GLT-1 and GS are mainly located in astrocytes, the changes of astrocytes were detected by glial fibrillary acidic protein (GFAP; an astrocytic marker) immunostaining. The results showed that BYHWD inhibited the expression of GFAP compared with the ischemia group, however, co-administration with PACAP-(6-38), which inhibited the effect of BYHWD on GLT-1 and GS in astrocytes, attenuated this effect, indicating that astrocytes participated in the protective role of BYHWD following focal ischemia. These results provided the evidence for the first time that not only neurons but also astrocytes contribute to the protective role of BYHWD, which opposes previous studies and may be a starting point for traditional medicine. | | | 26405540
 |
NFκB signaling drives pro-granulocytic astroglial responses to neuromyelitis optica patient IgG.
Walker-Caulfield, ME; Guo, Y; Johnson, RK; McCarthy, CB; Fitz-Gibbon, PD; Lucchinetti, CF; Howe, CL
Journal of neuroinflammation
12
185
2015
Show Abstract
Astrocytes expressing the aquaporin-4 water channel are a primary target of pathogenic, disease-specific immunoglobulins (IgG) found in patients with neuromyelitis optica (NMO). Immunopathological analyses of active NMO lesions highlight a unique inflammatory phenotype marked by infiltration of granulocytes. Previous studies characterized this granulocytic infiltrate as a response to vasculocentric complement activation and localized tissue destruction. In contrast, we observe that granulocytic infiltration in NMO lesions occurs independently of complement-mediated tissue destruction or active demyelination. These immunopathological findings led to the hypothesis that NMO IgG stimulates astrocyte signaling that is responsible for granulocytic recruitment in NMO.Histopathology was performed on archival formalin-fixed paraffin-embedded autopsy-derived CNS tissue from 23 patients clinically and pathologically diagnosed with NMO or NMO spectrum disorder. Primary murine astroglial cultures were stimulated with IgG isolated from NMO patients or control IgG from healthy donors. Transcriptional responses were assessed by microarray, and translational responses were measured by ELISA. Signaling through the NFκB pathway was measured by western blotting and immunostaining.Stimulation of primary murine astroglial cultures with NMO IgG elicited a reactive and inflammatory transcriptional response that involved signaling through the canonical NFκB pathway. This signaling resulted in the release of pro-granulocytic chemokines and was inhibited by the clinically relevant proteasome inhibitors bortezomib and PR-957.We propose that the astrocytic NFκB-dependent inflammatory response to stimulation by NMO IgG represents one of the earliest events in NMO pathogenesis, providing a target for therapeutic intervention upstream of irreversible cell death and tissue damage. | | | 26423139
 |
Evidence for a novel functional role of astrocytes in the acute homeostatic response to high-fat diet intake in mice.
Buckman, LB; Thompson, MM; Lippert, RN; Blackwell, TS; Yull, FE; Ellacott, KL
Molecular metabolism
4
58-63
2015
Show Abstract
Introduction of a high-fat diet to mice results in a period of voracious feeding, known as hyperphagia, before homeostatic mechanisms prevail to restore energy intake to an isocaloric level. Acute high-fat diet hyperphagia induces astrocyte activation in the rodent hypothalamus, suggesting a potential role of these cells in the homeostatic response to the diet. The objective of this study was to determine physiologic role of astrocytes in the acute homeostatic response to high-fat feeding.We bred a transgenic mouse model with doxycycline-inducible inhibition of NFkappaB (NFκB) signaling in astrocytes to determine the effect of loss of NFκB-mediated astrocyte activation on acute high-fat hyperphagia. ELISA was used to measure the levels of markers of astrocyte activation, glial-fibrillary acidic protein (GFAP) and S100B, in the medial basal hypothalamus.Inhibition of NFκB signaling in astrocytes prevented acute high-fat diet-induced astrocyte activation and resulted in a 15% increase in caloric intake (P less than 0.01) in the first 24 h after introduction of the diet.These data reveal a novel homeostatic role for astrocytes in the acute physiologic regulation of food intake in response to high-fat feeding. | | | 25685690
 |
Dysregulation of astrocyte extracellular signaling in Costello syndrome.
Krencik, R; Hokanson, KC; Narayan, AR; Dvornik, J; Rooney, GE; Rauen, KA; Weiss, LA; Rowitch, DH; Ullian, EM
Science translational medicine
7
286ra66
2015
Show Abstract
Astrocytes produce an assortment of signals that promote neuronal maturation according to a precise developmental timeline. Is this orchestrated timing and signaling altered in human neurodevelopmental disorders? To address this question, the astroglial lineage was investigated in two model systems of a developmental disorder with intellectual disability caused by mutant Harvey rat sarcoma viral oncogene homolog (HRAS) termed Costello syndrome: mutant HRAS human induced pluripotent stem cells (iPSCs) and transgenic mice. Human iPSCs derived from patients with Costello syndrome differentiated to astroglia more rapidly in vitro than those derived from wild-type cell lines with normal HRAS, exhibited hyperplasia, and also generated an abundance of extracellular matrix remodeling factors and proteoglycans. Acute treatment with a farnesyl transferase inhibitor and knockdown of the transcription factor SNAI2 reduced expression of several proteoglycans in Costello syndrome iPSC-derived astrocytes. Similarly, mice in which mutant HRAS was expressed selectively in astrocytes exhibited experience-independent increased accumulation of perineuronal net proteoglycans in cortex, as well as increased parvalbumin expression in interneurons, when compared to wild-type mice. Our data indicate that astrocytes expressing mutant HRAS dysregulate cortical maturation during development as shown by abnormal extracellular matrix remodeling and implicate excessive astrocyte-to-neuron signaling as a possible drug target for treating mental impairment and enhancing neuroplasticity. | Western Blotting | | 25947161
 |
Characterization of glioma stem-like cells from human glioblastomas.
Yamamuro, S; Okamoto, Y; Sano, E; Ochiai, Y; Ogino, A; Ohta, T; Hara, H; Ueda, T; Nakayama, T; Yoshino, A; Katayama, Y
International journal of oncology
47
91-6
2015
Show Abstract
Glioma stem-like cells (GSCs) could have potential for tumorigenesis, treatment resistance, and tumor recurrence (GSC hypothesis). However, the mechanisms underlying such potential has remained elusive and few ultrastructural features of the cells have been reported in detail. We therefore undertook observations of the antigenic characteristics and ultrastructural features of GSCs isolated from human glioblastomas. Tumor spheres formed by variable numbers of cells, exhibiting a variable appearance in both their size and shape, were frequently seen in GSCs expressing the stem cell surface markers CD133 and CD15. Increased cell nucleus atypia, mitochondria, rough endoplasmic reticulum, coated vesicles, and microvilli, were noted in the GSCs. Furthermore, cells at division phases and different phases of the apoptotic process were occasionally observed. These findings could imply that GSCs have certain relations with human neural stem cells (NSCs) but are primitively different from undifferentiated NSCs. The data may provide support for the GSC hypothesis, and also facilitate the establishment of future glioblastoma treatments targeting GSCs. | | | 25955568
 |
miR-26a and miR-384-5p are required for LTP maintenance and spine enlargement.
Gu, QH; Yu, D; Hu, Z; Liu, X; Yang, Y; Luo, Y; Zhu, J; Li, Z
Nature communications
6
6789
2015
Show Abstract
Long-term potentiation (LTP) is a form of synaptic plasticity that results in enhanced synaptic strength. It is associated with the formation and enlargement of dendritic spines-tiny protrusions accommodating excitatory synapses. Both LTP and spine remodelling are crucial for brain development, cognition and the pathophysiology of neurological disorders. The role of microRNAs (miRNAs) in the maintenance of LTP, however, is not well understood. Using next-generation sequencing to profile miRNA transcriptomes, we demonstrate that miR-26a and miR-384-5p specifically affect the maintenance, but not induction, of LTP and different stages of spine enlargement by regulating the expression of RSK3. Using bioinformatics, we also examine the global effects of miRNA transcriptome changes during LTP on gene expression and cellular activities. This study reveals a novel miRNA-mediated mechanism for gene-specific regulation of translation in LTP, identifies two miRNAs required for long-lasting synaptic and spine plasticity and presents a catalogue of candidate 'LTP miRNAs'. | | | 25858512
 |
Protein carbonylation after traumatic brain injury: cell specificity, regional susceptibility, and gender differences.
Lazarus, RC; Buonora, JE; Jacobowitz, DM; Mueller, GP
Free radical biology & medicine
78
89-100
2015
Show Abstract
Protein carbonylation is a well-documented and quantifiable consequence of oxidative stress in several neuropathologies, including multiple sclerosis, Alzheimer׳s disease, and Parkinson׳s disease. Although oxidative stress is a hallmark of traumatic brain injury (TBI), little work has explored the specific neural regions and cell types in which protein carbonylation occurs. Furthermore, the effect of gender on protein carbonylation after TBI has not been studied. The present investigation was designed to determine the regional and cell specificity of TBI-induced protein carbonylation and how this response to injury is affected by gender. Immunohistochemistry was used to visualize protein carbonylation in the brains of adult male and female Sprague-Dawley rats subjected to controlled cortical impact (CCI) as an injury model of TBI. Cell-specific markers were used to colocalize the presence of carbonylated proteins in specific cell types, including astrocytes, neurons, microglia, and oligodendrocytes. Results also indicated that the injury lesion site, ventral portion of the dorsal third ventricle, and ventricular lining above the median eminence showed dramatic increases in protein carbonylation after injury. Specifically, astrocytes and limited regions of ependymal cells adjacent to the dorsal third ventricle and the median eminence were most susceptible to postinjury protein carbonylation. However, these patterns of differential susceptibility to protein carbonylation were gender dependent, with males showing significantly greater protein carbonylation at sites distant from the lesion. Proteomic analyses were also conducted and determined that the proteins most affected by carbonylation in response to TBI include glial fibrillary acidic protein, dihydropyrimidase-related protein 2, fructose-bisphosphate aldolase C, and fructose-bisphosphate aldolase A. Many other proteins, however, were not carbonylated by CCI. These findings indicate that there is both regional and protein specificity in protein carbonylation after TBI. The marked increase in carbonylation seen in ependymal layers distant from the lesion suggests a mechanism involving the transmission of a cerebral spinal fluid-borne factor to these sites. Furthermore, this process is affected by gender, suggesting that hormonal mechanisms may serve a protective role against oxidative stress. | | | 25462645
 |
Expression of progerin in aging mouse brains reveals structural nuclear abnormalities without detectible significant alterations in gene expression, hippocampal stem cells or behavior.
Baek, JH; Schmidt, E; Viceconte, N; Strandgren, C; Pernold, K; Richard, TJ; Van Leeuwen, FW; Dantuma, NP; Damberg, P; Hultenby, K; Ulfhake, B; Mugnaini, E; Rozell, B; Eriksson, M
Human molecular genetics
24
1305-21
2015
Show Abstract
Hutchinson-Gilford progeria syndrome (HGPS) is a segmental progeroid syndrome with multiple features suggestive of premature accelerated aging. Accumulation of progerin is thought to underlie the pathophysiology of HGPS. However, despite ubiquitous expression of lamin A in all differentiated cells, the HGPS mutation results in organ-specific defects. For example, bone and skin are strongly affected by HGPS, while the brain appears to be unaffected. There are no definite explanations as to the variable sensitivity to progeria disease among different organs. In addition, low levels of progerin have also been found in several tissues from normal individuals, but it is not clear if low levels of progerin contribute to the aging of the brain. In an attempt to clarify the origin of this phenomenon, we have developed an inducible transgenic mouse model with expression of the most common HGPS mutation in brain, skin, bone and heart to investigate how the mutation affects these organs. Ultrastructural analysis of neuronal nuclei after 70 weeks of expression of the LMNA c.1824Cgreater than T mutation showed severe distortion with multiple lobulations and irregular extensions. Despite severe distortions in the nuclei of hippocampal neurons of HGPS animals, there were only negligible changes in gene expression after 63 weeks of transgenic expression. Behavioral analysis and neurogenesis assays, following long-term expression of the HGPS mutation, did not reveal significant pathology. Our results suggest that certain tissues are protected from functional deleterious effects of progerin. | | | 25343989
 |