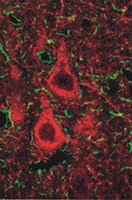
Loss of SED1/MFG-E8 results in altered luminal physiology in the epididymis.
Raymond, AS; Elder, B; Ensslin, M; Shur, BD
Molecular reproduction and development
77
550-63
2010
Show Abstract
SED1/MFG-E8, herein referred to as SED1, is a bimotif adhesive protein with ascribed functions in a range of cell-cell interactions, including sperm-egg binding. In the male reproductive tract, SED1 is secreted by the initial segment of the epididymis, where it coats sperm and subsequently facilitates binding to the egg zona pellucida. We have recently reported that SED1-null epididymides show an unexpected incidence of spermatic granulomas, reflecting breakdown of the epithelium and a consequent autoimmune response against sperm antigens. However, spermatic granulomas are most often manifest in the distal segments of the epididymis, whereas the bulk of SED1 is expressed in the proximal epididymis. In some models, the presence of granulomas in the distal epididymis is associated with an underlying defect in the maintenance of luminal fluid homeostasis. Herein, we report that SED1-null epididymal fluid is both hypo-osmotic and alkaline, relative to wildtype epididymal fluid. Furthermore, the SED1-null epididymal epithelium exhibits various hallmarks of disrupted fluid reabsorption and pH regulation, including altered morphology of clear cells, increased intracellular vesicles, and apical distribution of VATPase. Results indicate that the SED1-null epididymal pathologies are not the secondary consequences of defective testes or efferent ducts or of improper epididymal differentiation, unlike that seen in other epididymal models. The expression and distribution of various ion exchangers, channels, and enzymes that mediate fluid transport and pH regulation are examined in wildtype and SED1-null epididymides, and models to account for how SED1 functions in luminal fluid dynamics are discussed. | Immunohistochemistry | | 20422713
 |
Functional integration of embryonic stem cell-derived neurons in vivo.
Wernig, Marius, et al.
J. Neurosci., 24: 5258-68 (2004)
2004
Show Abstract
Pluripotency and the potential for continuous self-renewal make embryonic stem (ES) cells an attractive donor source for neuronal cell replacement. Despite recent encouraging results in this field, little is known about the functional integration of transplanted ES cell-derived neurons on the single-cell level. To address this issue, ES cell-derived neural precursors exhibiting neuron-specific enhanced green fluorescent protein (EGFP) expression were introduced into the developing brain. Donor cells implanted into the cerebral ventricles of embryonic rats migrated as single cells into a variety of brain regions, where they acquired complex morphologies and adopted excitatory and inhibitory neurotransmitter phenotypes. Synaptic integration was suggested by the expression of PSD-95 (postsynaptic density-95) on donor cell dendrites, which in turn were approached by multiple synaptophysin-positive host axon terminals. Ultrastructural and electrophysiological data confirmed the formation of synapses between host and donor cells. Ten to 21 d after birth, all EGFP-positive donor cells examined displayed active membrane properties and received glutamatergic and GABAergic synaptic input from host neurons. These data demonstrate that, at the single-cell level, grafted ES cell-derived neurons undergo morphological and functional integration into the host brain circuitry. Antibodies to the region-specific transcription factors Bf1, Dlx, En1, and Pax6 were used to explore whether functional donor cell integration depends on the acquisition of a regional phenotype. Our data show that incorporated neurons frequently exhibit a lacking or ectopic expression of these transcription factors. Thus, the lack of an appropriate regional "code" does not preclude morphological and synaptic integration of ES cell-derived neurons. | | | 15175396
 |
Modulation of glutamate transporters (GLAST, GLT-1 and EAAC1) in the rat cerebellum following portocaval anastomosis.
Suárez, I, et al.
Brain Res., 859: 293-302 (2000)
2000
Show Abstract
Glutamate transporters have the important function of removing glutamate released from synapses and keeping extracellular glutamate concentrations below excitotoxic levels. Extracellular glutamate increases in portocaval anastomosis (PCA), so we used a portacaval anastomosis model in rats to analyze the expression of glutamate transporters (GLAST, GLT-1 and EAAC1) in rat cerebellum, 1 and 6 months after PCA, using immunohistochemical methods. In controls, EAAC1 immunoreactivity in Purkinje cells and glial GLAST and GLT-1 immunoreactivities in the molecular layer (ML) increased from young to old rats. One month after PCA, Purkinje cell bodies were not immunostained for neuronal EAAC1 glutamate transporter, whereas glial glutamate transporter expressions (GLAST and GLT-1) were decreased when compared to young controls. In rats with long-term PCA (6 months post-PCA), neuronal and glial glutamate transporter expressions were increased. The expression of the neuronal glutamate transporter EAAC1 was less intense than old controls, whereas glial glutamate transporters (GLAST and GLT-1) increased more than their controls. Since the level of the neuronal glutamate transporter (EAAC1) in long-term PCA did not reach that of the controls, GLAST and GLT-1 glutamate transporters seemed to be required to ensure the glutamate uptake in this type of encephalopathy. EAAC1 immunoreactivity also was expressed by Bergmann glial processes in long-term PCA, but this increase did not suffice to reverse the alterations caused at the early stage. The present findings provide evidence that transitory alteration of glutamate transporter expressions could be a significant factor in the accumulation of excess glutamate in the extracellular space in PCA, which probably makes Purkinje cells more vulnerable to glutamate effect. | | | 10719077
 |
Mammalian ion-coupled solute transporters.
Hediger, M A, et al.
J. Physiol. (Lond.), 482: 7S-17S (1995)
1995
Show Abstract
Active transport of solutes into and out of cells proceeds via specialized transporters that utilize diverse energy-coupling mechanisms. Ion-coupled transporters link uphill solute transport to downhill electrochemical ion gradients. In mammals, these transporters are coupled to the co-transport of H+, Na+, Cl- and/or to the countertransport of K+ or OH-. By contrast, ATP-dependent transporters are directly energized by the hydrolysis of ATP. The development of expression cloning approaches to select cDNA clones solely based on their capacity to induce transport function in Xenopus oocytes has led to the cloning of several ion-coupled transporter cDNAs and revealed new insights into structural designs, energy-coupling mechanisms and physiological relevance of the transporter proteins. Different types of mammalian ion-coupled transporters are illustrated by discussing transporters isolated in our own laboratory such as the Na+/glucose co-transporters SGLT1 and SGLT2, the H(+)-coupled oligopeptide transporters PepT1 and PepT2, and the Na(+)- and K(+)-dependent neuronal and epithelial high affinity glutamate transporter EAAC1. Most mammalian ion-coupled organic solute transporters studied so far can be grouped into the following transporter families: (1) the predominantly Na(+)-coupled transporter family which includes the Na+/glucose co-transporters SGLT1, SGLT2, SGLT3 (SAAT-pSGLT2) and the inositol transporter SMIT, (2) the Na(+)- and Cl(-)-coupled transporter family which includes the neurotransmitter transporters of gamma-amino-butyric acid (GABA), serotonin, dopamine, norepinephrine, glycine and proline as well as transporters of beta-amino acids, (3) the Na(+)- and K(+)-dependent glutamate/neurotransmitter family which includes the high affinity glutamate transporters EAAC1, GLT-1, GLAST, EAAT4 and the neutral amino acid transporters ASCT1 and SATT1 reminiscent of system ASC and (4) the H(+)-coupled oligopeptide transporter family which includes the intestinal H(+)-dependent oligopeptide transporter PepT1. | | | 7730974
 |
Neuronal high-affinity glutamate transport in the rat central nervous system.
Kanai, Y, et al.
Neuroreport, 6: 2357-62 (1995)
1995
Show Abstract
EAAC1 is a neuronal and epithelial high affinity glutamate transporter previously cloned from rabbit intestine. Here we report the isolation of EAAC 1 from rat brain* and its expression in the central nervous system based on in situ hybridization. Strong signals were detected in brain, spinal cord and retina. Expression of EAAC1 was particularly strong in pyramidal cells of the cerebral cortex, pyramidal cells of the hippocampus, mitral cells of the olfactory bulb, various thalamic nuclei and cells of certain retinal layers. EAAC1 was also expressed in non-glutamatergic neurons such as GABAergic cerebellar Purkinje cells and alpha-motor neurons of the spinal cord. We propose that EAAC1 is not only involved in the sequestration of glutamate at glutamatergic synapses and in protecting neurons from glutamate excitotoxicity, but also in the cellular metabolism involving glutamate. | | Rat | 8747153
 |
Localization of neuronal and glial glutamate transporters.
Rothstein, J D, et al.
Neuron, 13: 713-25 (1994)
1994
Show Abstract
The cellular and subcellular distributions of the glutamate transporter subtypes EAAC1, GLT-1, and GLAST in the rat CNS were demonstrated using anti-peptide antibodies that recognize the C-terminal domains of each transporter. On immunoblots, the antibodies specifically recognize proteins of 65-73 kDa in total brain homogenates. Immunocytochemistry shows that glutamate transporter subtypes are distributed differentially within neurons and astroglia. EAAC1 is specific for certain neurons, such as large pyramidal cortical neurons and Purkinje cells, but does not appear to be selective for glutamatergic neurons. GLT-1 is localized only to astroglia. GLAST is found in both neurons and astroglia. The regional localizations are unique to each transporter subtype. EAAC1 is highly enriched in the cortex, hippocampus, and caudate-putamen and is confined to pre- and postsynaptic elements. GLT-1 is distributed in astrocytes throughout the brain and spinal cord. GLAST is most abundant in Bergmann glia in the cerebellar molecular layer brain, but is also present in the cortex, hippocampus, and deep cerebellar nuclei. | | | 7917301
 |
Primary structure and functional characterization of a high-affinity glutamate transporter.
Kanai, Y and Hediger, M A
Nature, 360: 467-71 (1992)
1992
Show Abstract
Glutamate transport across plasma membranes of neurons, glial cells and epithelial cells of the small intestine and kidney proceeds by high- and low-affinity transport systems. High-affinity (Km 2-50 microM) transport systems have been described that are dependent on Na+ but not Cl- ions and have a preference for L-glutamate and D- and L-aspartate. In neurons high-affinity glutamate transporters are essential for terminating the postsynaptic action of glutamate by rapidly removing released glutamate from the synaptic cleft. We have isolated a complementary DNA encoding an electrogenic Na(+)- but not Cl(-)-dependent high-affinity glutamate transporter (named EAAC1) from rabbit small intestine by expression in Xenopus oocytes. We find EAAC1 transcripts in specific neuronal structures in the central nervous system as well as in the small intestine, kidney, liver and heart. The function and pharmacology of the expressed protein are characteristic of the high-affinity glutamate transporter already identified in neuronal tissues. The abnormal glutamate transport that is associated with certain neurodegenerative diseases and which occurs during ischaemia and anoxia could be due to abnormalities in the function of this protein. | | | 1280334
 |