Detailed expression pattern of aldolase C (Aldoc) in the cerebellum, retina and other areas of the CNS studied in Aldoc-Venus knock-in mice.
Fujita, H; Aoki, H; Ajioka, I; Yamazaki, M; Abe, M; Oh-Nishi, A; Sakimura, K; Sugihara, I
PloS one
9
e86679
2014
Show Abstract
Aldolase C (Aldoc, also known as "zebrin II"), a brain type isozyme of a glycolysis enzyme, is expressed heterogeneously in subpopulations of cerebellar Purkinje cells (PCs) that are arranged longitudinally in a complex striped pattern in the cerebellar cortex, a pattern which is closely related to the topography of input and output axonal projections. Here, we generated knock-in Aldoc-Venus mice in which Aldoc expression is visualized by expression of a fluorescent protein, Venus. Since there was no obvious phenotypes in general brain morphology and in the striped pattern of the cerebellum in mutants, we made detailed observation of Aldoc expression pattern in the nervous system by using Venus expression in Aldoc-Venus heterozygotes. High levels of Venus expression were observed in cerebellar PCs, cartwheel cells in the dorsal cochlear nucleus, sensory epithelium of the inner ear and in all major types of retinal cells, while moderate levels of Venus expression were observed in astrocytes and satellite cells in the dorsal root ganglion. The striped arrangement of PCs that express Venus to different degrees was carefully traced with serial section alignment analysis and mapped on the unfolded scheme of the entire cerebellar cortex to re-identify all individual Aldoc stripes. A longitudinally striped boundary of Aldoc expression was first identified in the mouse flocculus, and was correlated with the climbing fiber projection pattern and expression of another compartmental marker molecule, heat shock protein 25 (HSP25). As in the rat, the cerebellar nuclei were divided into the rostrodorsal negative and the caudoventral positive portions by distinct projections of Aldoc-positive and negative PC axons in the mouse. Identification of the cerebellar Aldoc stripes in this study, as indicated in sample coronal and horizontal sections as well as in sample surface photos of whole-mount preparations, can be referred to in future experiments. | | | 24475166
 |
Transgenic overexpression of PKCε in the mouse prostate induces preneoplastic lesions.
Benavides, F; Blando, J; Perez, CJ; Garg, R; Conti, CJ; DiGiovanni, J; Kazanietz, MG
Cell cycle (Georgetown, Tex.)
10
268-77
2011
Show Abstract
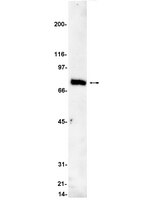
It is well established that protein kinase C (PKC) isozymes play distinctive roles in mitogenic and survival signaling as well as in cancer progression. PKCε, the product of the PRKCE gene, is up-regulated in various types of cancers including prostate, lung and breast cancer. To address a potential role for PKCs in prostate cancer progression we generated three mouse transgenic lines expressing PKCα, PKCδ, or PKCε in the prostate epithelium under the control of the rat probasin (PB) promoter. Whereas PB-PKCε and PB-PKCδ mice did not show any evident phenotype, PB-PKCε mice developed prostate hyperplasia as well as prostate intraepithelial neoplasia (PIN) that displayed enhanced phospho-Akt, phospho-S6, and phospho-Stat3 levels, as well as enhanced resistance to apoptotic stimuli. PKCε overexpression was insufficient to drive neoplastic changes in the mouse prostate. Notably, overexpression of PKCε by adenoviral means in normal immortalized RWPE-1 prostate cells confers a growth advantage and hyperactivation of Erk and Akt. Our results argue for a causal link between PKCε overexpression and prostate cancer development. Full Text Article | Western Blotting | Mouse | 21224724
 |
The Spalt family transcription factor Sall3 regulates the development of cone photoreceptors and retinal horizontal interneurons.
de Melo, J; Peng, GH; Chen, S; Blackshaw, S
Development (Cambridge, England)
138
2325-36
2011
Show Abstract
The mammalian retina is a tractable model system for analyzing transcriptional networks that guide neural development. Spalt family zinc-finger transcription factors play a crucial role in photoreceptor specification in Drosophila, but their role in mammalian retinal development has not been investigated. In this study, we show that that the spalt homolog Sall3 is prominently expressed in developing cone photoreceptors and horizontal interneurons of the mouse retina and in a subset of cone bipolar cells. We find that Sall3 is both necessary and sufficient to activate the expression of multiple cone-specific genes, and that Sall3 protein is selectively bound to the promoter regions of these genes. Notably, Sall3 shows more prominent expression in short wavelength-sensitive cones than in medium wavelength-sensitive cones, and that Sall3 selectively activates expression of the short but not the medium wavelength-sensitive cone opsin gene. We further observe that Sall3 regulates the differentiation of horizontal interneurons, which form direct synaptic contacts with cone photoreceptors. Loss of function of Sall3 eliminates expression of the horizontal cell-specific transcription factor Lhx1, resulting in a radial displacement of horizontal cells that partially phenocopies the loss of function of Lhx1. These findings not only demonstrate that Spalt family transcription factors play a conserved role in regulating photoreceptor development in insects and mammals, but also identify Sall3 as a factor that regulates terminal differentiation of both cone photoreceptors and their postsynaptic partners. Full Text Article | | | 21558380
 |
Differential distribution of exchange proteins directly activated by cyclic AMP within the adult rat retina.
C M Whitaker,N G F Cooper
Neuroscience
165
2010
Show Abstract
The recently discovered exchange protein directly activated by cAMP (Epac), a guanine exchange factor for the G-protein RAP-1, is directly activated by cAMP independently of protein kinase A (PKA). While cAMP is known to be an important second messenger in the retina, the presence of Epac has not been investigated in this tissue. The goal of the present study was to determine if the Epac1 and Epac2 genes are present and to characterize their location within the retina. Western blot analysis revealed that Epac1 and Epac2 proteins are expressed within the retina, and the presence of mRNA was demonstrated with the aid of reverse transcriptase polymerase chain reaction (RT-PCR). Additionally, we used immunofluorescence and confocal microscopy to demonstrate that Epac1 and Epac2 have overlapping as well as unique distributions within the retina. Both are present within horizontal cells, rod and cone bipolar cells, cholinergic amacrine cells, retrograde labeled retinal ganglion cells, and Müller cells. Uniquely, Epac2 was expressed by cone photoreceptor inner and outer segments, cell bodies, and synaptic terminals. In contrast, Epac1 was expressed in vesicular glutamate transporter 1 (VGlut1) and C-terminal binding protein 2 (CtBP2) positive photoreceptor synaptic terminals. Together, these results provide evidence that Epac1 and Epac2 are differentially expressed within the retina and provide the framework for further functional studies of cAMP pathways within the retina. Full Text Article | | | 19883736
 |
Different changes in protein and phosphoprotein levels result from serum starvation of high-grade glioma and adenocarcinoma cell lines.
Levin, VA; Panchabhai, SC; Shen, L; Kornblau, SM; Qiu, Y; Baggerly, KA
Journal of proteome research
9
179-91
2010
Show Abstract
Tumor cells undergoing serum starvation in vitro partially mimic metabolically stressed cells trying to adjust to a changed environment in vivo by inducing signal transduction and gene expression so that the tumor continues to grow. Our hypothesis is that the changes in protein and phosphoprotein levels after serum starvation may reflect the adapted phenotype of the tumor, which could be targeted for therapy. We used reverse-phase protein microarrays to interrogate five high-grade glioma cell lines and seven adenocarcinoma cell lines for differences in the level of 81 proteins and 25 phosphoproteins. All cell lines were studied in the well-fed condition of growth with 10% FBS and the starved condition of 0.5% FBS. Protein expression levels were normalized to beta-actin and trichotomized as increased (+1, upper 75th quartile), decreased (-1, lowest 25th quartile), or unchanged (0, others) to focus on the patterns of the biggest (and hopefully most robust) changes in protein and phosphoprotein levels. We examined these trichotomized values to better understand Starved-Fed differences among the cell lines and thereby gain better/clearer insight into the effects of serum starvation on potential cellular responses. In general, the expression of proteins and phosphoproteins 24 h after FBS starvation increased more often in glioma lines than in adenocarcinoma lines, which appeared to have fewer increased protein scores and more decreased scores. Many of the proteins increased in gliomas were downstream targets of the PTEN-PI-3 kinase-AKT, EGFR-MAPK-Stat, and transcription activator-polyamine signaling pathways. In adenocarcinomas, the expression of proteins and phosphoproteins generally increased in apoptosis pathways, while there were minor fluctuations in the other pathways above. Contrawise, gliomas become resistant to apoptosis after 24 h of serum starvation and upregulate transcription activators and polyamines more so than adenocarciomas. | Western Blotting | Human | 19894763
 |
The long noncoding RNA RNCR2 directs mouse retinal cell specification.
Rapicavoli, NA; Poth, EM; Blackshaw, S
BMC developmental biology
10
49
2010
Show Abstract
Recent work has identified that many long mRNA-like noncoding RNAs (lncRNAs) are expressed in the developing nervous system. Despite their abundance, the function of these ncRNAs has remained largely unexplored. We have investigated the highly abundant lncRNA RNCR2 in regulation of mouse retinal cell differentiation.We find that the RNCR2 is selectively expressed in a subset of both mitotic progenitors and postmitotic retinal precursor cells. ShRNA-mediated knockdown of RNCR2 results in an increase of both amacrine cells and Müller glia, indicating a role for this lncRNA in regulating retinal cell fate specification. We further report that RNCR2 RNA, which is normally nuclear-retained, can be exported from the nucleus when fused to an IRES-GFP sequence. Overexpression of RNCR2-IRES-GFP phenocopies the effects of shRNA-mediated knockdown of RNCR2, implying that forced mislocalization of RNCR2 induces a dominant-negative phenotype. Finally, we use the IRES-GFP fusion approach to identify specific domains of RNCR2 that are required for repressing both amacrine and Müller glial differentiation.These data demonstrate that the lncRNA RNCR2 plays a critical role in regulating mammalian retinal cell fate specification. Furthermore, we present a novel approach for generating dominant-negative constructs of lncRNAs, which may be generally useful in the functional analysis of this class of molecules. | Immunofluorescence | | 20459797
 |
A Technical Assessment of the Utility of Reverse Phase Protein Arrays for the Study of the Functional Proteome in Non-microdissected Human Breast Cancers.
Hennessy, BT; Lu, Y; Gonzalez-Angulo, AM; Carey, MS; Myhre, S; Ju, Z; Davies, MA; Liu, W; Coombes, K; Meric-Bernstam, F; Bedrosian, I; McGahren, M; Agarwal, R; Zhang, F; Overgaard, J; Alsner, J; Neve, RM; Kuo, WL; Gray, JW; Borresen-Dale, AL; Mills, GB
Clinical proteomics
6
129-51
2010
Show Abstract
The lack of large panels of validated antibodies, tissue handling variability, and intratumoral heterogeneity potentially hamper comprehensive study of the functional proteome in non-microdissected solid tumors. The purpose of this study was to address these concerns and to demonstrate clinical utility for the functional analysis of proteins in non-microdissected breast tumors using reverse phase protein arrays (RPPA).Herein, 82 antibodies that recognize kinase and steroid signaling proteins and effectors were validated for RPPA. Intraslide and interslide coefficients of variability were less than 15%. Multiple sites in non-microdissected breast tumors were analyzed using RPPA after intervals of up to 24 h on the benchtop at room temperature following surgical resection.Twenty-one of 82 total and phosphoproteins demonstrated time-dependent instability at room temperature with most variability occurring at later time points between 6 and 24 h. However, the 82-protein functional proteomic "fingerprint" was robust in most tumors even when maintained at room temperature for 24 h before freezing. In repeat samples from each tumor, intratumoral protein levels were markedly less variable than intertumoral levels. Indeed, an independent analysis of prognostic biomarkers in tissue from multiple tumor sites accurately and reproducibly predicted patient outcomes. Significant correlations were observed between RPPA and immunohistochemistry. However, RPPA demonstrated a superior dynamic range. Classification of 128 breast cancers using RPPA identified six subgroups with markedly different patient outcomes that demonstrated a significant correlation with breast cancer subtypes identified by transcriptional profiling.Thus, the robustness of RPPA and stability of the functional proteomic "fingerprint" facilitate the study of the functional proteome in non-microdissected breast tumors. Full Text Article | | | 21691416
 |
Developmental sources of conservation and variation in the evolution of the primate eye.
Michael A Dyer,Rodrigo Martins,Manoel da Silva Filho,José Augusto P C Muniz,Luiz Carlos L Silveira,Constance L Cepko,Barbara L Finlay
Proceedings of the National Academy of Sciences of the United States of America
106
2009
Show Abstract
Conserved developmental programs, such as the order of neurogenesis in the mammalian eye, suggest the presence of useful features for evolutionary stability and variability. The owl monkey, Aotus azarae, has developed a fully nocturnal retina in recent evolution. Description and quantification of cell cycle kinetics show that embryonic cytogenesis is extended in Aotus compared with the diurnal New World monkey Cebus apella. Combined with the conserved mammalian pattern of retinal cell specification, this single change in retinal progenitor cell proliferation can produce the multiple alterations of the nocturnal retina, including coordinated reduction in cone and ganglion cell numbers, increase in rod and rod bipolar numbers, and potentially loss of the fovea. Full Text Article | | | 19451636
 |
Role for protein kinase C-alpha in keratinocyte growth arrest.
Jerome-Morais, A; Rahn, HR; Tibudan, SS; Denning, MF
The Journal of investigative dermatology
129
2365-75
2009
Show Abstract
Multiple protein kinase C (PKC) isoforms have been associated with the epidermal keratinocyte (KC) granular layer differentiation program. Here we show PKCalpha membrane localization and substrate phosphorylation in the first suprabasal KCs of normal human epidermis, suggesting activation in vivo in the lower spinous layers where terminal differentiation-associated growth arrest occurs. To determine if PKCalpha is sufficient for KC growth arrest, we expressed a constitutively active PKCalpha (PKCalpha Delta22-28) in normal human KCs and observed growth arrest and accumulation of cells in G1. PKCalpha Delta22-28 inhibited DNA synthesis through the induction of the cyclin-dependent kinase inhibitor p21. Furthermore, downregulation of PKCalpha in an in vitro organotypic epidermis resulted in increased basal and suprabasal proliferation marker expression, decreased differentiation, and reduced epidermal stratification. Together these results indicate that PKCalpha activation is both necessary and sufficient to trigger irreversible growth arrest during human KC differentiation. | | | 19340015
 |
Phorbol ester-stimulated NF-kappaB-dependent transcription: roles for isoforms of novel protein kinase C.
Neil S Holden, Paul E Squires, Manminder Kaur, Rosemary Bland, Carol E Jones, Robert Newton
Cellular signalling
20
1338-48
2008
Show Abstract
Since protein kinase C (PKC) isoforms are variously implicated in the activation of NF-kappaB, we have investigated the role of PKC in the activation of NF-kappaB-dependent transcription by the diacyl glycerol (DAG) mimetic, phorbol 12-myristate 13-acetate (PMA), and by tumour necrosis factor (TNF) alpha in pulmonary A549 cells. The PKC selective inhibitors, Ro31-8220, Gö6976, GF109203X and Gö6983, revealed no effect on TNFalpha-induced NF-kappaB DNA binding and a similar lack of effect on serine 32/36 phosphorylated IkappaBalpha and the loss of total IkappaBalpha indicates that activation of the core IKK-IkappaBalpha-NF-kappaB cascade by TNFalpha does not involve PKC. In contrast, differential sensitivity of an NF-kappaB-dependent reporter to Ro31-8220, Gö6976, GF109203X and Gö6983 (EC(50)s 0.46 microM, 0.34 microM, >10 microM and >10 microM respectively) suggests a role for protein kinase D in transcriptional activation by TNFalpha. Compared with TNFalpha, PMA weakly induces NF-kappaB DNA binding and this effect was not associated with serine 32/36 phosphorylation of IkappaBalpha. However, PMA-stimulated NF-kappaB DNA binding was inhibited by Ro31-8220 (10 microM), GF109203X (10 microM) and Gö6983 (10 microM), but not by Gö6976 (10 microM), suggesting a role for novel PKC isoforms. Furthermore, a lack of positive effect of calcium mobilising agents on both NF-kappaB DNA binding and on transcriptional activation argues against major roles for classical PKCs. This, combined with the ability of both GF109203X and Gö6983 to prevent enhancement of TNFalpha-induced NF-kappaB-dependent transcription by PMA, further indicates a role for novel PKCs in NF-kappaB transactivation. Finally, siRNA-mediated knockdown of PKCdelta and epsilon expression did not affect TNFalpha-induced NF-kappaB-dependent transcription. However, knockdown of PKCdelta expression significantly inhibited PMA-stimulated luciferase activity, whereas knockdown of PKCepsilon was without effect. Furthermore, combined knockdown of PKCdelta and epsilon revealed an increased inhibitory effect on PMA-stimulated NF-kappaB-dependent transcription suggesting that PMA-induced NF-kappaB-dependent transcription is driven by novel PKC isoforms, particularly PKCdelta and epsilon. | | | 18436431
 |