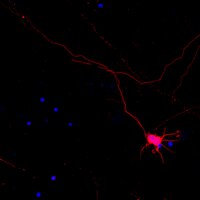
Loss of photoreceptors results in upregulation of synaptic proteins in bipolar cells and amacrine cells.
Dagar, S; Nagar, S; Goel, M; Cherukuri, P; Dhingra, NK
PloS one
9
e90250
2014
Show Abstract
Deafferentation is known to cause significant changes in the postsynaptic neurons in the central nervous system. Loss of photoreceptors, for instance, results in remarkable morphological and physiological changes in bipolar cells and horizontal cells. Retinal ganglion cells (RGCs), which send visual information to the brain, are relatively preserved, but show aberrant firing patterns, including spontaneous bursts of spikes in the absence of photoreceptors. To understand how loss of photoreceptors affects the circuitry presynaptic to the ganglion cells, we measured specific synaptic proteins in two mouse models of retinal degeneration. We found that despite the nearly total loss of photoreceptors, the synaptophysin protein and mRNA levels in retina were largely unaltered. Interestingly, the levels of synaptophysin in the inner plexiform layer (IPL) were higher, implying that photoreceptor loss results in increased synaptophysin in bipolar and/or amacrine cells. The levels of SV2B, a synaptic protein expressed by photoreceptors and bipolar cells, were reduced in whole retina, but increased in the IPL of rd1 mouse. Similarly, the levels of syntaxin-I and synapsin-I, synaptic proteins expressed selectively by amacrine cells, were higher after loss of photoreceptors. The upregulation of syntaxin-I was evident as early as one day after the onset of photoreceptor loss, suggesting that it did not require any massive or structural remodeling, and therefore is possibly reversible. Together, these data show that loss of photoreceptors results in increased synaptic protein levels in bipolar and amacrine cells. Combined with previous reports of increased excitatory and inhibitory synaptic currents in RGCs, these results provide clues to understand the mechanism underlying the aberrant spiking in RGCs. | 24595229
 |
Neuron-specific antigen HPC-1 from bovine brain reveals strong homology to epimorphin, an essential factor involved in epithelial morphogenesis: identification of a novel protein family.
Inoue, A and Akagawa, K
Biochem. Biophys. Res. Commun., 187: 1144-50 (1992)
1992
Show Abstract
We have already cloned the cDNA for the HPC-1 antigen, a neuron-specific protein antigen from the rat brain. Here we report the molecular cloning of the bovine HPC-1 antigen homologue, and much strong sequence conservation between rat and bovine. By searching the recent protein data base, it was found that the HPC-1 antigen revealed unusual similarity to epimorphin which was mesenchymal factor related to the morphogenesis of primitive epidermal tissues in embryonic stages. We also found that the HPC-1 antigen was identical to p35A (syntaxin) which bound both to a synaptic vesicle protein and to N-type calcium channel. Although the relationship of the physiological functions, structures and topologies along cellular membrane between the HPC-1 antigen and epimorphin have not been consistent yet, these two proteins belong to a novel protein family. | 1530610
 |