The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction.
Mortimer, L; Moreau, F; Cornick, S; Chadee, K
PLoS pathogens
11
e1004887
2015
Show Abstract
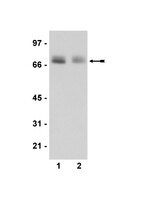
Entamoeba histolytica (Eh) is an extracellular protozoan parasite of humans that invades the colon to cause life-threatening intestinal and extra-intestinal amebiasis. Colonized Eh is asymptomatic, however, when trophozoites adhere to host cells there is a considerable inflammatory response that is critical in the pathogenesis of amebiasis. The host and/or parasite factors that trigger the inflammatory response to invading Eh are not well understood. We recently identified that Eh adherence to macrophages induces inflammasome activation and in the present study we sought to determine the molecular events upon contact that coordinates this response. Here we report that Eh contact-dependent activation of α5β1 integrin is critical for activation of the NLRP3 inflammasome. Eh-macrophage contact triggered recruitment of α5β1 integrin and NLRP3 into the intercellular junction, where α5β1 integrin underwent activation by an integrin-binding cysteine protease on the parasite surface, termed EhCP5. As a result of its activation, α5β1 integrin induced ATP release into the extracellular space through opening of pannexin-1 channels that signalled through P2X7 receptors to deliver a critical co-stimulatory signal that activated the NLRP3 inflammasome. Both the cysteine protease activity and integrin-binding domain of EhCP5 were required to trigger α5β1 integrin that led to ATP release and NLRP3 inflammasome activation. These findings reveal engagement of α5β1 integrin across the parasite-host junction is a key regulatory step that initiates robust inflammatory responses to Eh. We propose that α5β1 integrin distinguishes Eh direct contact and functions with NLRP3 as pathogenicity sensor for invasive Eh infection. | 25955828
 |
Paxillin: adapting to change.
Brown, Michael C and Turner, Christopher E
Physiol. Rev., 84: 1315-39 (2004)
2004
Show Abstract
Molecular scaffold or adaptor proteins facilitate precise spatiotemporal regulation and integration of multiple signaling pathways to effect the optimal cellular response to changes in the immediate environment. Paxillin is a multidomain adaptor that recruits both structural and signaling molecules to focal adhesions, sites of integrin engagement with the extracellular matrix, where it performs a critical role in transducing adhesion and growth factor signals to elicit changes in cell migration and gene expression. | 15383653
 |
The interplay between Src and integrins in normal and tumor biology.
Playford, Martin P and Schaller, Michael D
Oncogene, 23: 7928-46 (2004)
2004
Show Abstract
Src family nonreceptor protein tyrosine kinases transduce signals that control normal cellular processes such as cell proliferation, adhesion and motility. Normally, cellular Src is held in an inactive state, but in several cancer types, abnormal events lead to elevated kinase activity of the protein and cause pleiotropic cellular responses inducing transformation and metastasis. A prerequisite of the ability of a cancer cell to undergo metastasis into distant tissues is to penetrate surrounding extracellular matrices. These processes are facilitated by the integrin family of cell adhesion molecules. As is the case with Src, altered integrin activity or substrate affinity can contribute to the neoplastic phenotype. Therefore, understanding the interplay between Src and integrin function has been of intense interest over the past few years. This review focuses on the role of Src and integrin signaling in normal cells and how this is deregulated in human cancer. We will identify the key players in the integrin-mediated signaling pathways involved in cell motility and apoptosis, such as FAK, paxillin and p130(CAS), and discuss how Src signaling affects the formation of focal adhesions and the extracellular matrix. | 15489911
 |
FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration?
Schaller, Michael D
J. Cell Biol., 166: 157-9 (2004)
2004
Show Abstract
FAK and paxillin are important components in integrin-regulated signaling. New evidence suggests that these two proteins function in crosstalk between cell-matrix and cell-cell adhesions. Further, new insight suggests that under some conditions these proteins inhibit cell migration, in contrast to their established roles in several cell systems as positive regulators of cell adhesion and migration. | 15263014
 |
Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration.
Panetti, Tracee S
Front. Biosci., 7: d143-50 (2002)
2002
Show Abstract
Integrins are transmembrane receptors that mediate cell attachment to the substrate. At the cytoplasmic surface of the integrin, cytoskeletal proteins cluster into focal adhesions. The focal adhesions contain multiple proteins that provide a structural and signaling complex inside the cell. This review focuses on three of the cytoskeletal components of the focal adhesion, paxillin, FAK, and p130CAS, that are phosphorylated and play a regulatory role in cell spreading and cell migration. A brief discussion is included of tyrosine phosphorylation of the integrin in relation to localization and phosphorylation of these cytoskeletal proteins. The phosphorylation of integrins and cytoskeletal proteins regulates localization and downstream signaling with profound effects on cell movement. | 11779709
 |