Multiplex Assays for COVID-19 (SARS-CoV-2) Research Applications: Multiplex Your COVID-19 Research
- What Proteins Make Up the SARS-CoV-2 Virus?
- Multiplex Assays for Detection of Human Immunoglobulins Against SARS-CoV-2 Antigens
- What Is A Cytokine Storm?
- How Does Cytokine Storm Relate to COVID-19 (SARS-CoV-2)?
- Multiplex Assays for Cytokine Storm-Related COVID-19 Research
- Multiplex Assays for Emerging COVID-19 Research Areas
- COVID-19 On-Demand Webinars Featuring MILLIPLEX® Kits
- Select Published COVID-19 Studies Using MILLIPLEX® Kits
- References
Read on to learn how multiplex immunoassays, such as MIILIPLEX® multiplex assays, offer researchers the ability to simultaneously quantitate a large number of human cytokines, chemokines, and growth factors, and detect human immunoglobulins against SARS-CoV-2 antigens to better understand the immune response to COVID-19, including cytokine storm. Also, see how these multiplex biomarker assays can help explore emerging areas of SARS-CoV-2 research, such as angiogenesis and coagulopathy.
What Proteins Make Up the SARS-CoV-2 Virus?
Multiple proteins make up the SARS-CoV-2 virus. The spike (S) proteins that form the “corona” of the virus are composed of the subunit S1, which contains the receptor binding domain (RBD), and subunit S2. The spikes surround the membrane glycoprotein (M) and envelope protein (E) which contain the viral RNA encased by the nucleocapsid (N) protein (Figure 1).

Figure 1.Antigenic proteins of the SARS-CoV-2 coronavirus.
The SARS-CoV-2 viral RBD protein binds to the human angiotensin-converting enzyme 2 (ACE-2) receptors of cells found in multiple organs including the lungs, heart, arteries, gut, and kidneys. Once bound, the virus enters the cell, replicates, and is released to continue the infection cycle.
Each of these viral proteins is a potential antigen against which the immune system can form antibodies to fight infection. The earliest antibodies to appear are immunoglobulin A (IgA) which forms in the mucosal tissues of the nasal passages and gut, and the humoral immunoglobulin M (IgM). The humoral immunoglobulin G (IgG) forms later and can confer lasting immunity to disease. All three immunoglobulins can be measured in blood serum and plasma samples.
Multiplex Assays for Detection of Human Immunoglobulins Against SARS-CoV-2 Antigens
By testing COVID-19 patient serum/plasma sample immunoglobulin response to SARS-CoV-2 antigens, researchers may identify individuals who have been exposed to the SARS-CoV-2 virus and have generated some level of immune response. Researchers may further understand the immune response to the virus over the course of infection and recovery from COVID-19. Multiplex assays, such as MILLIPLEX® SARS-CoV-2 multiplex research assays, enable the detection of human immunoglobulins against SARS-CoV-2 antigens. These configurable 4-plex panels allow researchers to select any or all of the following SARS-CoV-2 viral antigens:
- Spike S1
- Spike S2
- RBD
- N protein
See examples of how researchers are using these multiplex panels below.
Example Data
EDTA plasma samples were assayed in duplicate for all four analytes, S1, S2, RBD, and N, according to the protocol in each of the MILLIPLEX® SARS-CoV-2 multiplex kits, IgG, IgA, and IgM. Samples were from patient groups testing positive or negative by PCR for SARS-CoV-2 infection: COVID-19 positive “Ventilated” (n=68), COVID-19 positive “Not Ventilated” (n=115), and COVID-19 negative “COVID-” (n=41).1 Graphed assay results (Figure 2) show individual MFI for each sample in each group with group means, +/- standard deviation (SD), and p-value significance tests between groups: ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05.
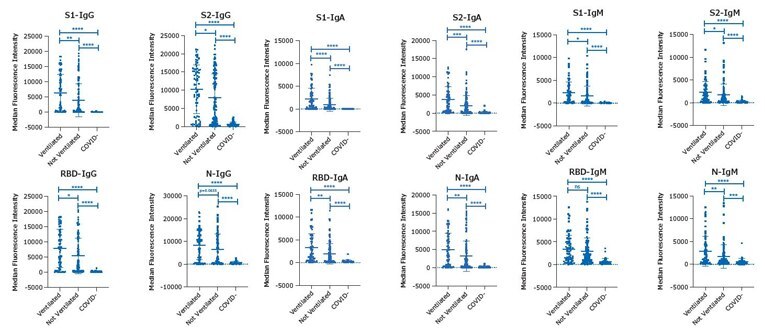
Figure 2.Data for all four analytes, S1, S2, RBD, and N with the MILLIPLEX® SARS-CoV-2 kits, IgG, IgA, and IgM using EDTA plasma samples.
Read the Application Note highlighting our MILLIPLEX® multiplex research assays to detect antibodies that recognize SARS-CoV-2 antigenic proteins in human serum and plasma.
MILLIPLEX® SARS-CoV-2 Multiplex Kits
For Research Use Only. Not For Use In Diagnostic Procedures.
What Is A Cytokine Storm?
A cytokine storm is when the immune system overreacts to a pathogen or other immunogenic substance such as a drug, a hyperinflammatory response may trigger excess production of signaling molecules from immune cells. This is referred to as cytokine storm syndrome (CSS) or cytokine release syndrome (CRS). Acute or systemic inflammation results in fluid buildup in the lungs, respiratory distress, multiple organ failure, and can be fatal.
How Does Cytokine Storm Relate to COVID-19 (SARS-CoV-2)?
In response to SARS-CoV-2 viral infection of the lungs, a cytokine storm can result. Over-produced immune cells and their signaling molecules cause a local inflammatory response in the lungs leading to respiratory distress and reduced blood oxygen levels. A cytokine storm can contribute to severe clinical symptoms and poor patient outcomes.
Some early publications on the cytokine profile for COVID-19 have found increased levels of IL- 2, IL-7, G-CSF, IP-10, MCP-1, MIP-1α, TNFα, and Ferritin.2 In a separate study, IL-6 was also found to increase with SARS-CoV-2 infection.3 Tocilizumab, an immunosuppressive monoclonal antibody therapy that targets the IL-6 receptor (IL-6R) has been approved for Phase III clinical trials by the FDA for the treatment of COVID-19 pneumonia as of March 26, 2020. IL-1β, IL- 1RA, IL-8, IL-9, IL-10, FGF-basic, GM-CSF, IFNγ, MIP-1β, PDGF, and VEGF have also been shown to be increased in COVID-19 patients compared to healthy subjects.4
Multiplex Assays for Cytokine Storm-Related COVID-19 Research
Much research has been done on the relationship between cytokine storm and COVID-19, in both human and non-human primate studies.
Human Studies
Recent publications on the cytokine profile in COVID-19 have found elevated levels of M-CSF, IP-10, IL-1RA, IL-10, IL-15, IL-27, TNFα, and IL-8 were predictive of cytokine storm or correlated with more severe disease.5,6 In a profiling study, it was found that circulating levels of IL-6, IL-8, TNFα, and IL-10 were associated with severe COVID-19, in addition to IL-18, IP-10, and MIG, among others.7
Non-Human Primate Studies
Research on SARS-CoV-2 is also being conducted in non-human primates such as rhesus macaques, which will allow researchers to test possible vaccines and antiviral medications/treatments in relevant animal models. A recent Nature publication used MILLIPLEX® Non-Human Primate multiplex assays to analyze serum over multiple time points in macaques with SARS-CoV-2 exposure for changes in cytokine and chemokine levels and showed increases in IL-1RA, IL-6, IL-10, IL-15, MCP-1, MIP-1β, along with a decrease in TGFα.8
Our MILLIPLEX® Non-Human Primate Cytokine/Chemokine/Growth Factor Panel A allows for simultaneous quantification of 48 immune factors in serum, plasma, and cell/tissue culture supernatant samples. Choose any combination of analytes, customize your premix, or select a 38-plex or 48-plex premixed kit.
Using MILLIPLEX® Multiplex Immunoassays to Understand the Immune Response to COVID-19
Our MILLIPLEX® multiplex immunoassays offer researchers the ability to simultaneously quantitate numerous analytes critical to understanding the immune response in humans. Our 48-Plex Human Cytokine/Chemokine/Growth Factor Panel A saves time and sample volume for a snapshot of analyte profiles during a cytokine storm, sepsis, or other disease states (Figure 3).
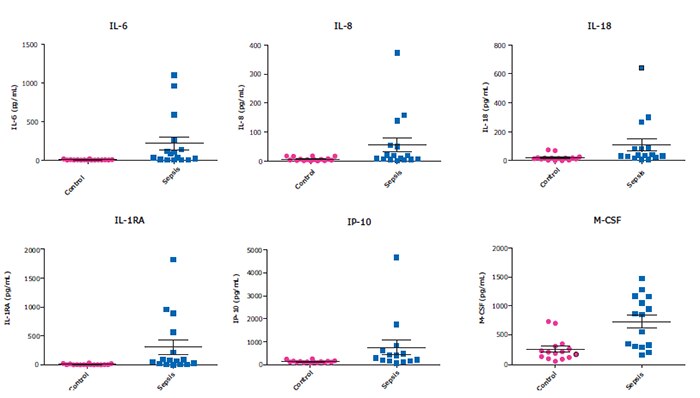
Figure 3.Healthy control (n=20) and sepsis patients (n=16) serum/plasma samples (obtained from BioIVT, Discovery, and BioChemed) were tested neat (25 µL/well) in the HCYTA-60K panel. Shown here is a subset of the analytes which have been mentioned in recent publications to be increased in SARS-CoV-2 cytokine release syndrome (CRS).
Related MILLIPLEX® Kits
We offer a wide array of MILLIPLEX® soluble protein panels and cell signaling kits to help elucidate the downstream signaling pathways when researching antiviral immune response. Our portfolio offers the widest range of analytes across the most species, including non-human primate panels for vaccine research.
For Research Use Only. Not For Use In Diagnostic Procedures.
Multiplex Assays for Emerging COVID-19 Research Areas
ACE and ACE2 play a role in the plasma kallikrein-kinin system (KKS), which controls the blood coagulation system, endothelial cell growth, angiogenesis, the complement pathway, and the renin angiotensin system (RAS).9 Bradykinin, a kinin peptide, along with kallikreins and kininogens make up the KKS. ACE2 functions to decrease bradykinin, and when disrupted, higher bradykinin levels can increase systemic inflammation.10 In addition to CSS, the disruption of the RAS/KKS may lead to severe complications in COVID-19. Moreover, the activation of bradykinin receptors mediates inflammation, leading to distinctly elevated cytokine levels. Our MILLIPLEX® multiplex immunoassays offer researchers the ability to simultaneously quantitate numerous analytes critical to understanding these mechanisms triggered by COVID-19, including complement components in our Human Complement Expanded Panel 1 and Human Complement Panel 2, Renin, Kallikrein-6, and our Human Angiogenesis/Growth Factor Panel 1 and Human Angiogenesis Panel 2.
Coagulopathy is a severe condition that has been associated with COVID-19, indicated by elevated D-dimer levels and extensive microthrombosis in lung autopsies.11 Thrombotic complications are associated with multiorgan failure and a higher rate of mortality. Higher levels of acute phase proteins, such as CRP and fibrinogen, have been shown to correlate with this COVID-19 severity. Increased cytokine levels, a hallmark of disease noted previously, can lead to upregulation of adhesion molecules such as ICAM-1, VCAM-1, P-selectin, and e-Selectin.12 With our MILLIPLEX® Human Cardiovascular Disease Panel 2, ICAM-1, VCAM-1, D-Dimer, and P-selectin can be quantitated simultaneously to study this critical condition.
Related MILLIPLEX® Assays
Your research breakthroughs depend on reliable, high-performing products and services. Our MILLIPLEX® portfolio of immunoassays is the largest portfolio of multiplex biomarker assays, based on Luminex® xMAP® technology, offering you consistent, high-quality results, so you can do your best work while saving time, labor, and cost.
For Research Use Only. Not For Use In Diagnostic Procedures.
COVID-19 On-Demand Webinars Featuring MILLIPLEX® Kits
View our on-demand webinars demonstrating how MILLIPLEX® multiplex assays have been used in COVID-19 research. In these webinars, you will discover how the immune response in COVID-19 patients is assessed and how to successfully integrate multiplex immunoassays into your disease research workflow.
- IgG Antibodies Against SARS-CoV-2 Correlate to Days from Symptom Onset in COVID-19 Positive Patients
- Keeping COVID-19 at Bay (only available in the U.S. and Canada)
- The Dynamics of SARS-CoV-2 Specific Antibody Responses in COVID-19 Patients (not available in the U.S. and Canada)
- Immune Responses in Severe COVID-19 Patients (not available in the U.S. and Canada)
- MILLIPLEX® Multiplex Immunoassays For Use in COVID-19 and Cytokine Storm Research
For Research Use Only. Not For Use In Diagnostic Procedures.
Did you use MILLIPLEX® products in your paper?
How to Cite Our Products in Your Paper
Ready to publish? We’re happy you’ve used our products to help you move forward in your research!
How to cite MILLIPLEX® assay kits:
- If you are based in the United States or Canada, state MilliporeSigma as the source of your assay.
- If you are based anywhere in the world outside of the United States or Canada, state Merck KGaA, Darmstadt, Germany, as the source of your assay.
- Include the full MILLIPLEX® kit name and kit catalog number, and also list the analytes you used from the kit.
- Include the species of samples you used and how you diluted your samples.
References
To continue reading please sign in or create an account.
Don't Have An Account?