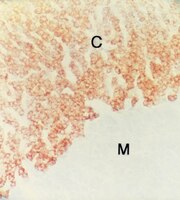
Areolae of the placenta in the Antarctic minke whale (Balaenoptera bonaerensis).
Sasaki, M; Amano, Y; Hayakawa, D; Tsubota, T; Ishikawa, H; Mogoe, T; Ohsumi, S; Tetsuka, M; Miyamoto, A; Fukui, Y; Budipitojo, T; Kitamura, N
The Journal of reproduction and development
60
62-7
2014
概要を表示する
In this study, we examined the existence and structure of areolae and the steroidogenesis of areolar trophoblast cells in the Antarctic minke whale placenta morphologically and immunohistochemically. Placentas were collected from the 15th, 16th and 18th Japanese Whale Research Program under Special Permit in the Antarctic (JARPA) and 1st JARPA II organized by the Institute of Cetacean Research in Tokyo, Japan. The opening and cavity of fetal areolae formed by taller columnar trophoblast cells (areolar trophoblast cells) with long microvilli and a bright cytoplasm, as compared with the trophoblast cells of the chorionic villi interdigitating with the endometrial crypts, were recognized in observations of serial sections. The opening of the areolar cavity was hidden by chorionic villi with areolar trophoblast cells. Furthermore, a closed pouch-like structure lined by tall columnar cells similar to areolar trophoblast cells within the stroma of chorionic villi was noticed and continued to the areolar cavity, with the opening seen on serial sections. In a surface investigation of the chorion and endometrium by SEM, maternal (endometrial) areolae irregularly surrounded by endometrial folds were obvious. Moreover, we distinguished areolar trophoblast cells with long microvilli attached with many blebs from trophoblast cells. In our immunohistochemical observations, a steroidogenic enzyme, cytochrome P450 side chain cleavage enzyme (P450scc), was detected with strong immunoreactivity in trophoblast cells. However, areolar trophoblast cells showed weak or no immunoreactivity for P450scc. | Immunohistochemistry | 24351524
 |
FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development.
Guasti, L; Candy Sze, WC; McKay, T; Grose, R; King, PJ
Molecular and cellular endocrinology
371
182-8
2013
概要を表示する
Developmental signalling pathways are implicated in the formation and maintenance of the adrenal gland, but their roles are currently not well defined. In recent years it has emerged that Sonic hedgehog (Shh) and Wnt/β catenin signalling are crucial for the growth and development of the adrenal cortex. Here we demonstrate that Fibroblast growth factor receptor (Fgfr) 2 isoforms IIIb and IIIc are expressed mainly in the adrenal subcapsule during embryogenesis and that specific deletion of the Fgfr2 IIIb isoform impairs adrenal development, causing reduced adrenal growth and impaired expression of SF1 and steroidogenic enzymes. The hypoplastic adrenals also have thicker, disorganised capsules which retain Gli1 expression but no longer express Dlk1. Fgfr2 ligands were detected in both the capsule and the cortex, suggesting the importance of signalling between the capsule and the cortex in adrenal development. | Immunohistochemistry | 23376610
 |
Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice.
Anjum, S; Krishna, A; Sridaran, R; Tsutsui, K
Journal of experimental zoology. Part A, Ecological genetics and physiology
317
630-44
2012
概要を表示する
The changes in distribution and concentration of neuropeptides, gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin, and gonadotropin-releasing hormone receptor (GnRH-R) were evaluated and compared with reproductive parameters, such as cytochrome P450 side-chain cleavage (P450 SCC) enzyme activity, androgen receptors (AR) in the testis and serum testosterone levels, from birth to senescence in mice. The results showed the localization of these molecules mainly in the interstitial and germ cells as well as showed significant variations in immunostatining from birth to senescence. It was found that increased staining of testicular GnRH-R coincided with increased steroidogenic activity during pubertal and adult stages, whereas decreased staining coincides with decreased steroidogenic activity during senescence. Similar changes in immunostaining were confirmed by Western/slot blot analysis. Thus, these results suggest a putative role of GnRH during testicular pubertal development and senescence. Treatment with a GnRH agonist ([DTrp(6), Pro(9)-NEt] GnRH) to mice from prepubertal to pubertal period showed a significant increase in steroidogenic activity of the mouse testis and provided further support to the role of GnRH in testicular pubertal maturation. The significant decline in GnRH-R during senescence may be due to a significant increase in GnIH synthesis during senescence causing the decrease in GnRH-R expression. It is considered that significant changes in the levels of GnRH-R may be responsible for changes in steroidogenesis that causes either pubertal activation or senescence in testis of mice. Furthermore, changes in the levels of GnRH-R may be modulated by interactions among GnRH, GnIH, and kisspeptin in the testis. | | 23027641
 |
BLTK1 murine Leydig cells: a novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants.
Forgacs, AL; Ding, Q; Jaremba, RG; Huhtaniemi, IT; Rahman, NA; Zacharewski, TR
Toxicological sciences : an official journal of the Society of Toxicology
127
391-402
2012
概要を表示する
Leydig cells are the primary site of androgen biosynthesis in males. Several environmental toxicants target steroidogenesis resulting in both developmental and reproductive effects including testicular dysgenesis syndrome. The aim of this study was to evaluate the effect of several structurally diverse endocrine disrupting compounds (EDCs) on steroidogenesis in a novel BLTK1 murine Leydig cell model. We demonstrate that BLTK1 cells possess a fully functional steroidogenic pathway that produces low basal levels of testosterone (T) and express all the necessary steroidogenic enzymes including Star, Cyp11a1, Cyp17a1, Hsd3b1, Hsd17b3, and Srd5a1. Recombinant human chorionic gonadotropin (rhCG) and forskolin (FSK) elicited concentration- and time-dependent induction of 3',5'-cyclic adenosine monophosphate, progesterone (P), and T, as well as the differential expression of Star, Hsd3b6, Hsd17b3, and Srd5a1 messenger RNA levels. The evaluation of several structurally diverse male reproductive toxicants including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), atrazine, prochloraz, triclosan, monoethylhexyl phthalate (MEHP), glyphosate, and RDX in BLTK1 cells suggests different modes of action perturb steroidogenesis. For example, prochloraz and triclosan antifungals reduced rhCG induction of T, consistent with published in vivo data but did not alter basal T levels. In contrast, atrazine and MEHP elicited modest induction of basal T but antagonized rhCG-mediated induction of T levels, whereas TCDD, glyphosate, and RDX had no effect on basal or rhCG induction of T in BLTK1 cells. These results suggest that BLTK1 cells maintain rhCG-inducible steroidogenesis and are a viable in vitro Leydig cell model to evaluate the effects of EDCs on steroidogenesis. This model can also be used to elucidate the different mechanisms underlying toxicant-mediated disruption of steroidogenesis. | Western Blotting | 22461451
 |
Differential stimulation pathways of progesterone secretion from newly formed corpora lutea in rats treated with ethylene glycol monomethyl ether, sulpiride, or atrazine.
Taketa, Y; Yoshida, M; Inoue, K; Takahashi, M; Sakamoto, Y; Watanabe, G; Taya, K; Yamate, J; Nishikawa, A
Toxicological sciences : an official journal of the Society of Toxicology
121
267-78
2011
概要を表示する
Ethylene glycol monomethyl ether (EGME), sulpiride, and atrazine are known ovarian toxicants, which increase progesterone (P4) secretion and induce luteal cell hypertrophy following repeated administration. The aim of this study was to define the pathways by which these compounds exerted their effects on the ovary and hypothalamic-pituitary-gonadal (HPG) axis. In the ovary, changes in the steroidogenic activity of new and old corpora lutea (CL) were addressed. EGME (300 mg/kg), sulpiride (100 mg/kg), or atrazine (300 mg/kg) were orally given daily for four times from proestrus to diestrus in normal cycling rats. Treatment with all chemicals significantly increased serum P4 levels, and EGME as well as sulpiride induced increases in prolactin (PRL) levels. In new CL, at both the gene and the protein levels, all three chemicals upregulated the following steroidogenic factors: scavenger receptor class B type I, steroidogenic acute regulatory protein, P450 cholesterol side-chain cleavage, and 3β-hydroxysteroid dehydrogenase (HSD) and downregulated the luteolytic gene, 20α-HSD. Coadministration of EGME and bromocriptine, a D2 agonist, completely inhibited PRL but not P4 secretion. Additionally, steroidogenic factor expression levels were upregulated, and 20α-HSD level was downregulated in new CL. These results suggest that EGME both directly and indirectly stimulates P4 production in luteal cells, whereas sulpiride elevates P4 through activation of PRL secretion in the pituitary. Atrazine may directly activate new CL by stimulating steroidogenic factor expressions. The present study suggests that multiple pathways mediate the effects of EGME, sulpiride, and atrazine on the HPG axis and luteal P4 production in female rats in vivo. | | 21427058
 |
Complex ovarian defects lead to infertility in Etv5-/- female mice.
Eo, J; Shin, H; Kwon, S; Song, H; Murphy, KM; Lim, JH
Molecular human reproduction
17
568-76
2011
概要を表示する
Etv5 is a member of the Etv4 subfamily of Ets transcription factors. In female mice, Etv5 was previously shown to be expressed in the mouse ovary. In this work, we show that Etv5-/- female mice are infertile due to a complex reproductive phenotype. Defects in the ovarian tissue architecture were noted as early as 2 weeks of age in Etv5-/- mice. Adult Etv5-/- female mice show decreased ovulation and no interest in mating even after gonadotrophin treatment. Histological abnormalities were also noted in Etv5-/- ovaries. Injection of 17β-estradiol to gonadotrophin-treated Etv5-/- mice significantly increased ovulation, mating and fertilization rates. However, 2-cell embryos of Etv5-/- females show compromised development, suggesting a role for Etv5 in the developmental competence of embryos. Expression of aromatase (CYP11a1), 17α-hydroxylase/17,20 lyase/17,20 desmolase (CYP17a1), side-chain-cleaving enzyme (CYP19a1) and luteinizing hormone/choriogonadotropin receptor mRNAs was not significantly altered in Etv5-/- ovaries. Collectively, our results suggest that Etv5 is important for the developmental competence of germ cells and the regulation of responses to steroid hormones in female mice. | | 21478265
 |
Cyclic GMP signaling in rat urinary bladder, prostate, and epididymis: tissue-specific changes with aging and in response to Leydig cell depletion.
Müller, D; Mukhopadhyay, AK; Davidoff, MS; Middendorff, R
Reproduction (Cambridge, England)
142
333-43
2011
概要を表示する
Aging of the male reproductive system leads to changes in endocrine signaling and is frequently associated with the emergence of prostate hyperplasia and bladder dysfunctions. Recent reports highlight prostate and bladder as promising targets for therapeutic interventions with inhibitors of the cyclic GMP (cGMP)-degrading phosphodiesterase 5 (PDE5). However, the cGMP signaling system in these organs is as yet poorly characterized, and the possibility of age-related alterations has not been addressed. This study investigates key proteins of cGMP pathways in bladder, prostate, and epididymis of young (3 months) and old (23-24 months) Wistar rats. Local differences in the abundance of PDE5, soluble guanylyl cyclase (sGC) and particulate guanylyl cyclases (GC-A, GC-B), endothelial nitric oxide synthase, and cGMP-dependent protein kinase I (PRKG1 (cGKI)) revealed pronounced tissue-specific peculiarities. Although cGMP-generating enzymes were not affected by age in all organs, we recognized age-related decreases of PDE5 expression in bladder and a selective diminishment of membrane-associated PRKG1 in epididymis. In disagreement with published data, all cGMP pathway proteins including PDE5 are poorly expressed in prostate. However, prostatic PRKG1 expression increases with aging. Androgen withdrawal during temporary Leydig cell elimination induced a massive (greater than 12-fold) upregulation of PRKG1 in prostate but not in other (penis and epididymis) androgen-dependent organs. These findings identify PRKG1 as a key androgen-sensitive signaling protein in prostate of possible importance for growth regulation. The elucidated effects may have significance for age-associated pathologies in the male lower-urinary tract. | | 21511885
 |
The Leydig cell MEK/ERK pathway is critical for maintaining a functional population of adult Leydig cells and for fertility.
Yamashita, S; Tai, P; Charron, J; Ko, C; Ascoli, M
Molecular endocrinology (Baltimore, Md.)
25
1211-22
2011
概要を表示する
MAPK kinase (MEK)1 and MEK2 were deleted from Leydig cells by crossing Mek1(f/f);Mek2(-/-) and Cyp17iCre mice. Primary cultures of Leydig cell from mice of the appropriate genotype (Mek1(f/f);Mek2(-/-);iCre(+)) show decreased, but still detectable, MEK1 expression and decreased or absent ERK1/2 phosphorylation when stimulated with epidermal growth factor, Kit ligand, cAMP, or human choriogonadotropin (hCG). The body or testicular weights of Mek1(f/f);Mek2(-/-);iCre(+) mice are not significantly affected, but the testis have fewer Leydig cells. The Leydig cell hypoplasia is paralleled by decreased testicular expression of several Leydig cell markers, such as the lutropin receptor, steroidogenic acute regulatory protein, cholesterol side chain cleavage enzyme, 17α-hydroxylase, and estrogen sulfotransferase. The expression of Sertoli or germ cell markers, as well as the shape, size, and cellular composition of the seminiferous tubules, are not affected. cAMP accumulation in response to hCG stimulation in primary cultures of Leydig cells from Mek1(f/f);Mek2(-/-);iCre(+) mice is normal, but basal testosterone and testosterone syntheses provoked by addition of hCG or a cAMP analog, or by addition of substrates such as 22-hydroxycholesterol or pregnenolone, are barely detectable. The Mek1(f/f);Mek2(-/-);iCre(+) males show decreased intratesticular testosterone and display several signs of hypoandrogenemia, such as elevated serum LH, decreased expression of two renal androgen-responsive genes, and decreased seminal vesicle weight. Also, in spite of normal sperm number and motility, the Mek1(f/f);Mek2(-/-);iCre(+) mice show reduced fertility. These studies show that deletion of MEK1/2 in Leydig cells results in Leydig cell hypoplasia, hypoandrogenemia, and reduced fertility. | | 21527500
 |
Meiotic functions of RAD18.
Inagaki, A; Sleddens-Linkels, E; Wassenaar, E; Ooms, M; van Cappellen, WA; Hoeijmakers, JH; Seibler, J; Vogt, TF; Shin, MK; Grootegoed, JA; Baarends, WM
Journal of cell science
124
2837-50
2011
概要を表示する
RAD18 is an ubiquitin ligase that is involved in replication damage bypass and DNA double-strand break (DSB) repair processes in mitotic cells. Here, we investigated the testicular phenotype of Rad18-knockdown mice to determine the function of RAD18 in meiosis, and in particular, in the repair of meiotic DSBs induced by the meiosis-specific topoisomerase-like enzyme SPO11. We found that RAD18 is recruited to a specific subfraction of persistent meiotic DSBs. In addition, RAD18 is recruited to the chromatin of the XY chromosome pair, which forms the transcriptionally silent XY body. At the XY body, RAD18 mediates the chromatin association of its interaction partners, the ubiquitin-conjugating enzymes HR6A and HR6B. Moreover, RAD18 was found to regulate the level of dimethylation of histone H3 at Lys4 and maintain meiotic sex chromosome inactivation, in a manner similar to that previously observed for HR6B. Finally, we show that RAD18 and HR6B have a role in the efficient repair of a small subset of meiotic DSBs. | | 21807948
 |
Effect of chronic treatment with Rosiglitazone on Leydig cell steroidogenesis in rats: in vivo and ex vivo studies.
JA Couto, KL Saraiva, CD Barros, DP Udrisar, CA Peixoto, JS Vieira, MC Lima, SL Galdino, IR Pitta, MI Wanderley
Reproductive biology and endocrinology : RB&E
8
13
2010
概要を表示する
BACKGROUND: The present study was designed to examine the effect of chronic treatment with rosiglitazone - thiazolidinedione used in the treatment of type 2 diabetes mellitus for its insulin sensitizing effects - on the Leydig cell steroidogenic capacity and expression of the steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme (P450scc) in normal adult rats. METHODS: Twelve adult male Wistar rats were treated with rosiglitazone (5 mg/kg) administered by gavage for 15 days. Twelve control animals were treated with the vehicle. The ability of rosiglitazone to directly affect the production of testosterone by Leydig cells ex vivo was evaluated using isolated Leydig cells from rosiglitazone-treated rats. Testosterone production was induced either by activators of the cAMP/PKA pathway (hCG and dbcAMP) or substrates of steroidogenesis [22(R)-hydroxy-cholesterol (22(R)-OH-C), which is a substrate for the P450scc enzyme, and pregnenolone, which is the product of the P450scc-catalyzed step]. Testosterone in plasma and in incubation medium was measured by radioimmunoassay. The StAR and P450scc expression was detected by immunocytochemistry. RESULTS: The levels of total circulating testosterone were not altered by rosiglitazone treatment. A decrease in basal or induced testosterone production occurred in the Leydig cells of rosiglitazone-treated rats. The ultrastructural and immunocytochemical analysis of Leydig cells from rosiglitazone-treated rats revealed cells with characteristics of increased activity as well as increased StAR and P450scc expression, which are key proteins in androgen biosynthesis. However, a number of rosiglitazone-treated cells exhibited significant mitochondrial damage. CONCLUSION: The results revealed that the Leydig cells from rosiglitazone-treated rats showed significant reduction in testosterone production under basal, hCG/dbcAMP- or 22 (R)-OH-C/pregnenolone-induced conditions, although increased labeling of StAR and P450scc was detected in these cells by immunocytochemistry. The ultrastructural study suggested that the lower levels of testosterone produced by these cells could be due to mitochondrial damage induced by rosiglitazone., 記事全文 | | 20144211
 |