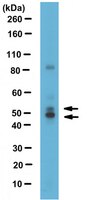
Calcineurin regulates nuclear factor I dephosphorylation and activity in malignant glioma cell lines.
Brun, M; Glubrecht, DD; Baksh, S; Godbout, R
The Journal of biological chemistry
288
24104-15
2013
概要を表示する
Malignant gliomas (MG), including grades III and IV astrocytomas, are the most common adult brain tumors. These tumors are highly aggressive with a median survival of less than 2 years. Nuclear factor I (NFI) is a family of transcription factors that regulates the expression of glial genes in the developing brain. We have previously shown that regulation of the brain fatty acid-binding protein (B-FABP; FABP7) and glial fibrillary acidic protein (GFAP) genes in MG cells is dependent on the phosphorylation state of NFI, with hypophosphorylation of NFI correlating with GFAP and B-FABP expression. Importantly, NFI phosphorylation is dependent on phosphatase activity that is enriched in GFAP/B-FABP+ve cells. Using chromatin immunoprecipitation, we show that NFI occupies the GFAP and B-FABP promoters in NFI-hypophosphorylated GFAP/B-FABP+ve MG cells. NFI occupancy, NFI-dependent transcriptional activity, and NFI phosphorylation are all modulated by the serine/threonine phosphatase calcineurin. Importantly, a cleaved form of calcineurin, associated with increased phosphatase activity, is specifically expressed in NFI-hypophosphorylated GFAP/B-FABP+ve MG cells. Calcineurin in GFAP/B-FABP+ve MG cells localizes to the nucleus. In contrast, calcineurin is primarily found in the cytoplasm of GFAP/B-FABP-ve cells, suggesting a dual mechanism for calcineurin activation in MG. Finally, our results demonstrate that calcineurin expression is up-regulated in areas of high infiltration/migration in grade IV astrocytoma tumor tissue. Our data suggest a critical role for calcineurin in NFI transcriptional regulation and in the determination of MG infiltrative properties. | Western Blotting | 23839947
 |
Chromatin-dependent cooperativity between site-specific transcription factors in vivo.
Hebbar, Pratibha B and Archer, Trevor K
J. Biol. Chem., 282: 8284-91 (2007)
2007
概要を表示する
Accessing binding sites in DNA wrapped around histones in condensed chromatin is an obstacle that transcription factors must overcome to regulate gene expression. Here we demonstrate cooperativity between two transcription factors, the glucocorticoid receptor (GR) and nuclear factor 1 (NF1) to bind the mouse mammary tumor virus promoter organized as regular chromatin in vivo. This cooperativity is not observed when the promoter is introduced transiently into cells. Using RNA interference to deplete NF1 protein levels in the cells, we confirmed that NF1 promotes binding of GR to the promoter. Furthermore, we observed a similar synergism between GR and NF1 binding on the endogenous 11beta-hydroxysteroid dehydrogenase promoter, also regulated by GR and NF1. Our results suggest that the chromatin architecture of the promoters does not permit strong association of GR in the absence of NF1. Therefore we propose that cooperativity among DNA binding factors in binding to their cognate recognition sites in chromatin may be an important feature in the regulation of gene expression. | | 17186943
 |
The p53 tumor suppressor gene is regulated in vivo by nuclear factor 1-C2 in the mouse mammary gland during pregnancy.
Johansson, Eva M, et al.
Oncogene, 22: 6061-70 (2003)
2003
概要を表示する
The p53 tumor suppressor protein plays an important role in preventing cancer development by arresting or killing potential tumor cells. Downregulated p53 levels, or mutations within the p53 gene, leading to the loss of p53 activity, are found in many breast carcinomas. Here we demonstrate that the p53 gene is transcriptionally upregulated in the normal mouse mammary gland at midpregnancy. We show that the specific isoform nuclear factor 1-C2 (NF1-C2) plays an important role in this activation. Functional mutation of the NF1-binding site in the mouse p53 promoter resulted in a reduction of the gene expression to less than 30% in mammary epithelial cells. By the use of two powerful techniques, chromatin immunoprecipitation and oligonucleotide decoy, we verify the importance of NF1-C2 in p53 gene activation in vivo. These findings demonstrate a broader role for NF1-C2 in the mammary gland at midpregnancy, beyond its earlier reported activation of milk protein genes. We also demonstrate that NF1-A1 proteins are produced in the mouse mammary gland. However, due to their lower affinity to the NF1-binding site, these proteins are not involved in the transcriptional upregulation of p53 at midpregnancy. This paper constitutes the first report demonstrating the importance of NF1 proteins in the p53 gene activation in the mouse mammary gland. It is also the first time that p53 gene activation is coupled to a specific, endogenously expressed NF1 isoform. | | 12955085
 |
Nuclear factor 1-C2 contributes to the tissue-specific activation of a milk protein gene in the differentiating mammary gland.
Kannius-Janson, Marie, et al.
J. Biol. Chem., 277: 17589-96 (2002)
2002
概要を表示する
Members of the nuclear factor 1 (NF1) transcription factor family have been postulated to be involved in the regulation of milk genes. In this work we have been able to identify the splice variant NF1-C2 as an important member of a tissue-specific activating complex that regulates the milk gene encoding carboxyl ester lipase (CEL). Mutation of the NF1-binding site in the CEL gene promoter results in a drastic reduction of the gene expression to about 15% in mammary epithelial cells. Furthermore, we demonstrate that the NF1-C2 protein interacts with a higher affinity to the NF1-binding site in the CEL gene promoter than other NF1 family members do and that NF1-C2 in the mouse mammary gland is a phosphorylated protein. During development of the mouse mammary gland, binding of NF1-C2 to the CEL gene promoter is induced at midpregnancy, in correlation with the induction of CEL gene expression. The fact that the NF1-C2 involving complex remains throughout the lactation period and decreases during the weaning period, when the CEL gene is down-regulated, supports its importance in the regulation of CEL gene expression. To our knowledge, this is the first report identifying a specific, endogenously expressed NF1 isoform to be involved in the tissue-specific activation of a gene. | | 11877413
 |