Cross Coupling
A cross-coupling reaction in organic synthesis occurs when two fragments are joined together with the aid of a metal catalyst. Cross-coupling has been an essential reaction in catalytic chemistry for the past 30 years starting with the pioneering work by Heck, Negishi, and Suzuki, who were awarded the Nobel Prize in Chemistry in 2010 for palladium-catalyzed cross-coupling. As one of the most versatile and powerful bond-forming methods in synthetic organic chemistry, the field of cross-coupling has matured to the point where nearly any two fragments can be coupled with the right catalyst. With its usage increasing exponentially, the field has grown to include numerous strategies for carbon-carbon, carbon-nitrogen, and carbon-oxygen bond formation, including key reactions such as:
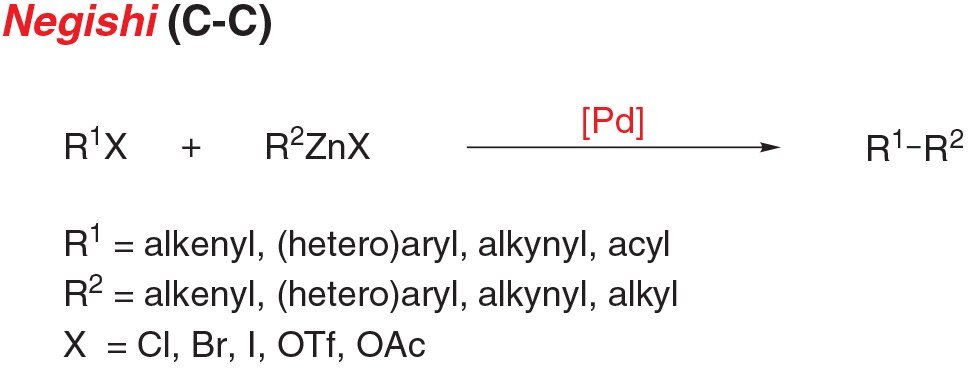
- Negishi coupling is the cross-coupling of an aryl (pseudo)halide and an organozinc nucleophile to form C-C bonds.
- Suzuki-Miyaura coupling, the cross-coupling of an aryl (pseudo)halide and organoborate, is a versatile reaction for carbon-carbon bond formation.
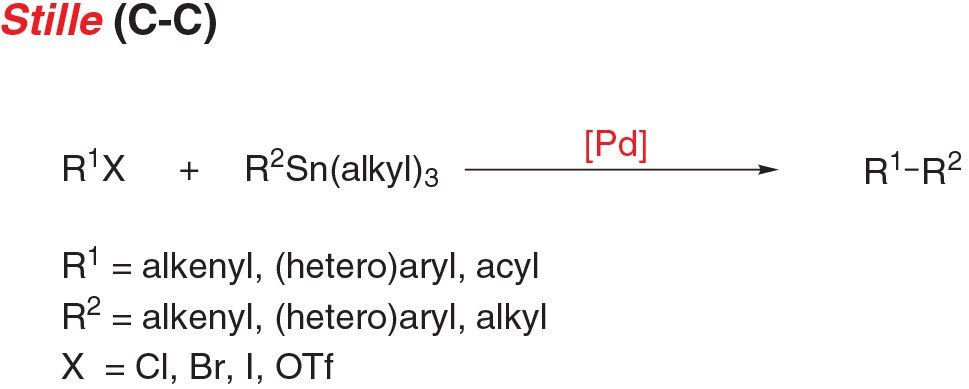
- Stille coupling, the cross coupling of an aryl (pseudo)halide and stannanes, is a functional reaction for carbon-carbon bond formation with few limitations on the R-groups.
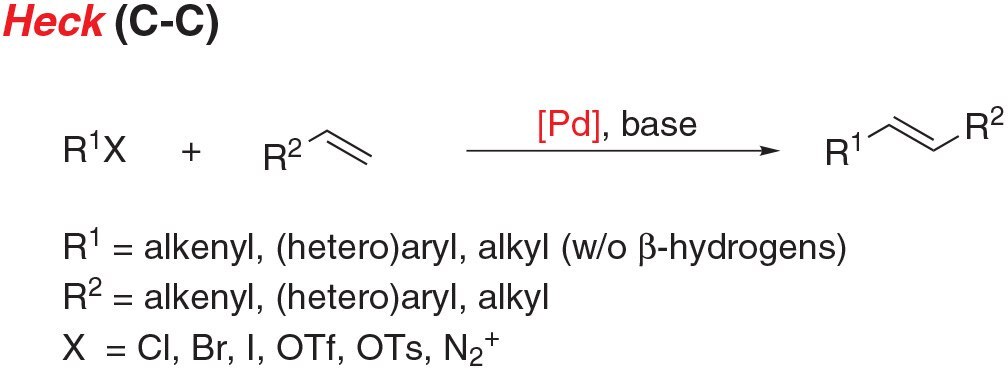
- Heck coupling is the cross coupling of an unsaturated halide with an alkene to give substituted alkenes.
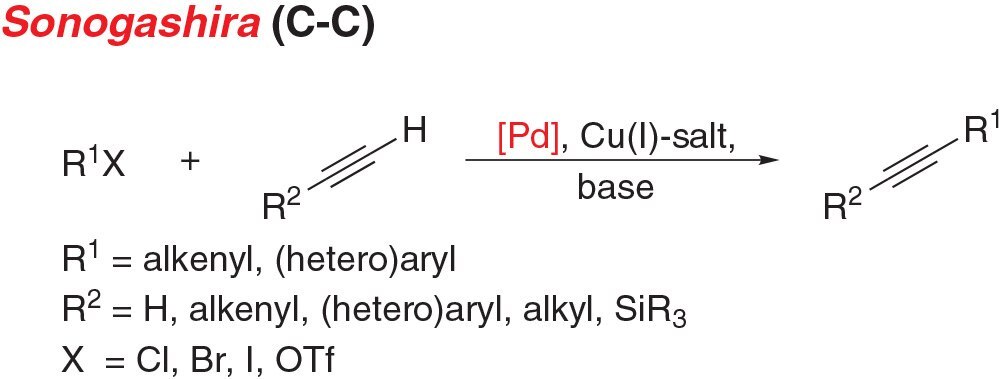
- Sonogashira coupling is the cross-coupling of an aryl (pseudo) halide with a terminal alkyne to give disubstituted acetylenes.
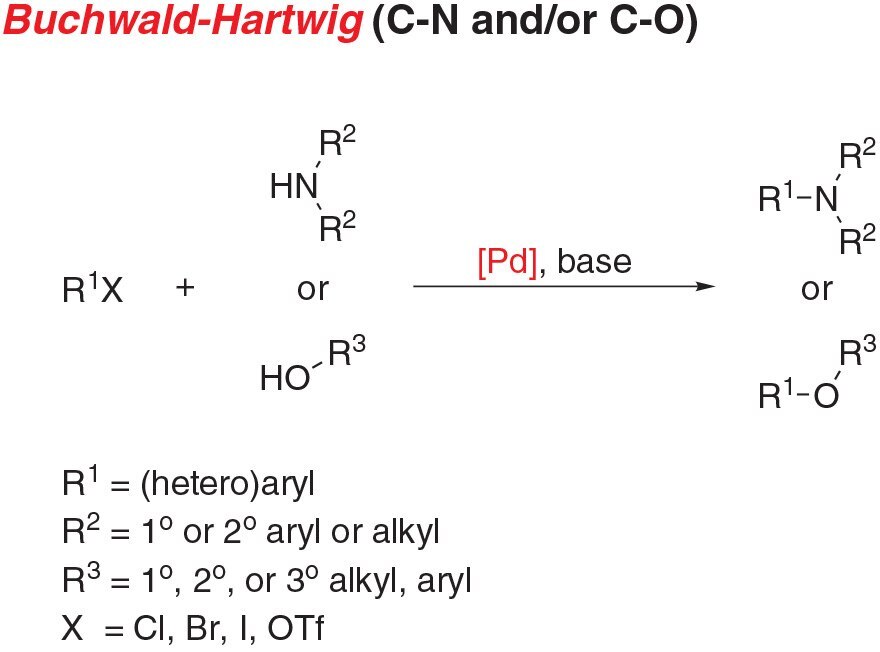
- Buchwald-Hartwig amination, the cross-coupling of an aryl (pseudo)halide and an amine, is a staple reaction for chemists across a wide range of disciplines.
Related Technical Articles
- All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
- Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
- A recyclable, ligand-free ruthenium catalyst for C–H activation reactions and concomitant C–C bond formation in the presence of water.
- 2-picoline-borane (pic-BH3) is an excellent alternative reagent for reductive aminations.
- Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
- See All (106)
Related Protocols
- Protocol for the KitAlysis™ Buchwald-Hartwig Amination Reaction Screening Kit
- Solutions & slurries to make: Go to the online user set-up page to enter molecular weights into the downloadable excel file.
- TPGS-750-M, a second generation surfactant, may be used for Suzuki-Miyaura Reactions in Water at Room Temperature
- TPGS-750-M, a second generation surfactant, may be used in the Buchwald-Hartwig Amination Reaction in Water at Room Temperature.
- Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
- See All (9)
To continue reading please sign in or create an account.
Don't Have An Account?