A Universal Plate-Based Immunoaffinity LC-MS/MS Workflow for Preclinical Monoclonal Antibody Quantification
Kevin Ray, Yue Lu, Pegah Jalili, Jeffrey Turner, Nicolas Caffarelli, Tom Juehne
MilliporeSigma, a business of Merck KGaA, Darmstadt, Germany
Introduction
Monoclonal antibodies are the largest class of biotherapeutics approved for a variety of clinical indications, particularly in oncology and autoimmune diseases. During preclinical drug development, assays are required to understand the absorption, distribution, metabolism and excretion of monoclonal antibodies, which is crucial for their design and selection. Hereby, we demonstrate a robust, high-throughput workflow for quantification of human IgG1 antibodies in animal sera by LC-MS/MS utilizing a stable isotope labeled universal monoclonal antibody internal standard which is introduced prior to immunoaffinity enrichment and tryptic digestion on an easy-to-use 96-well plate based kit format.
Methods
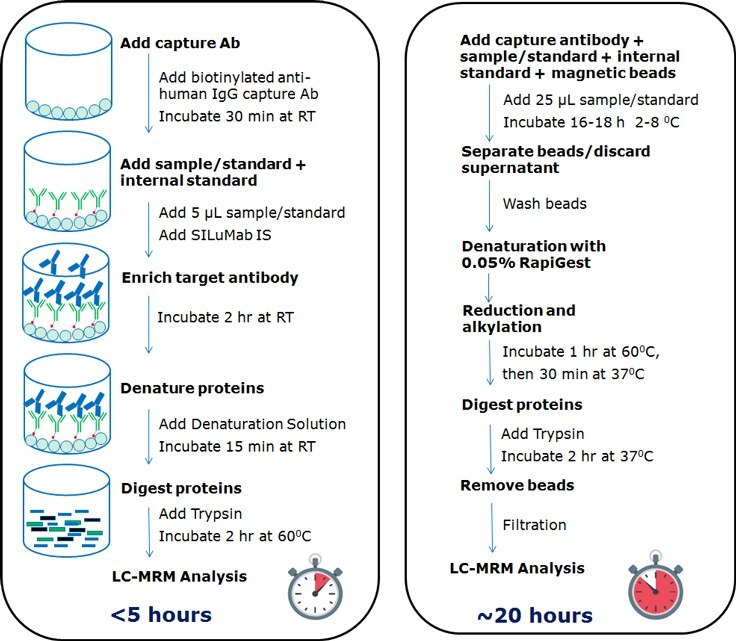
Figure 1. Comparison of universal plate-based and magnetic bead-based workflow for quantification IgG1 antibodies.
Results
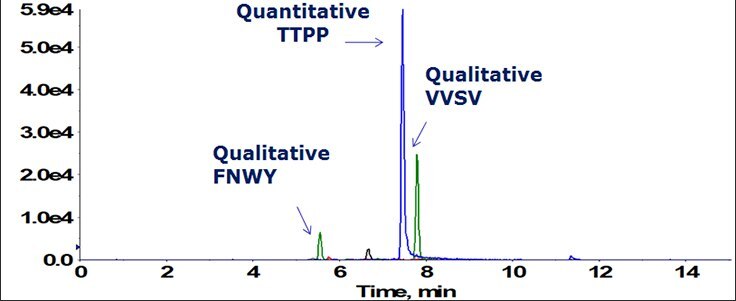
Figure 2. Total ion chromatogram of three selected signature peptides, one quantitative (TTPP) and two qualitative (VVSV and FNWY), used for universal analysis of IgG1 therapeutic drug antibodies in preclinical studies
Calibration Curves
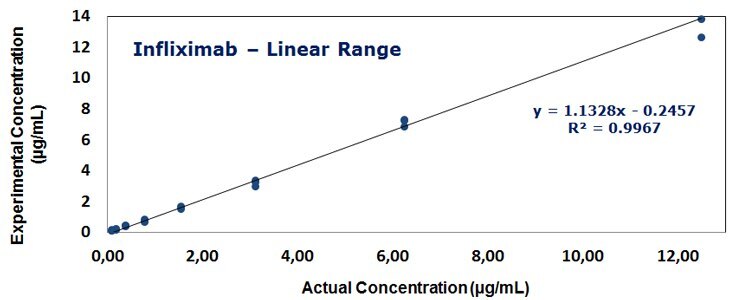
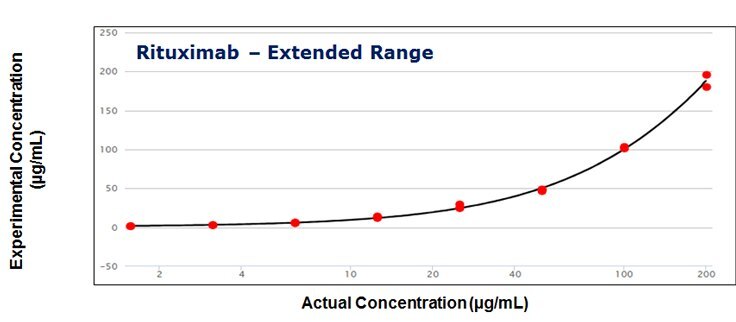
Figure 3. The calibration curves for standards of Infliximab over the concentration range of 0.1-12.5 μg/mL and Rituximab over the extended range of 0.8 - 200 μg/mL using 5PL regression.
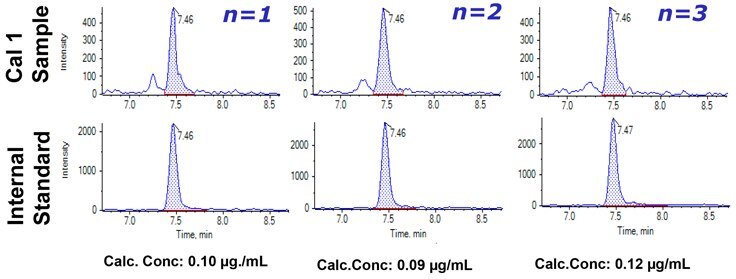
Figure 4. Reproducibility of universal TTPP signature peptide from Adalimumab at LLOQ of 0.1 μg/mL concentration.
Conclusions
- We have developed a plated-based kit which enables high-throughput quantification of human IgG1 antibodies in animal sera by LC-MS/MS in less than five hours.
- Universal assay performance was verified with several commercially available biotherapeutic antibodies, including Adalimumab, Infliximab, Rituximab and Cetuximab.
- The linear assay range was established in the range of 0.1 to 12.5 μg/mL using just 5 μl serum sample volume. The upper assay range could be extended to 200 μg/mL using a non-linear 5PL regression analysis.
- The calibration curves show a regression coefficient of >0.99, CV values of <20%, and accuracies ranging from 80-120%.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?