HPLC determination of Besifloxacin in Ophthalmic Suspension using Chromolith® SpeedROD RP-18e Column
Dr. Ajay Kaparwan, Dr. Sanjay Poman
, Mumbai Analytical Laboratory, Navi Mumbai, India
Scope
Here we demonstrate an application for Besifloxacin in ophthalmic suspension on RP-HPLC using Chromolith® SpeedROD RP-18e column for system suitability, linearity, repeatability, percentage recovery, LOD and LOQ.
Introduction
Besifloxacin (Figure 1) is a bactericidal fluoroquinolone-type antibiotic, used for the treatment of bacterial conjunctivitis caused by susceptible bacteria. It was approved by US FDA in May 2009. Here, a solution is demonstrated for the determination of Besifloxacin in ophthalmic suspension dosage using HPLC with a monolith column and UV Detection at 289 nm. At present, there are no pharmacopeial monographs available for the assay of Besifloxacin in ophthalmic suspension dosage forms.
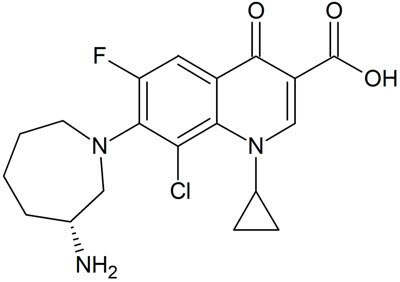
Figure 1.Chemical Structure of Besifloxacin
Instrumentation
- Waters Alliance™ e2695 with Empower 3 Software
- Milli-Q® Integral 3 Water purification system
- Ultrasonic bath from PCI analytics
- Millex® HV Durapore membrane filter (PVDF) 0.45 µm
Chromatographic Data of Besifloxacin Analysis
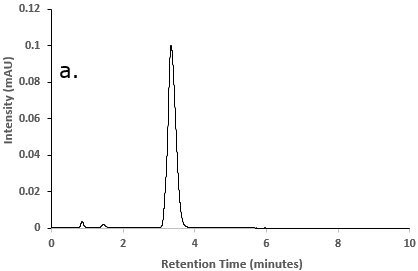
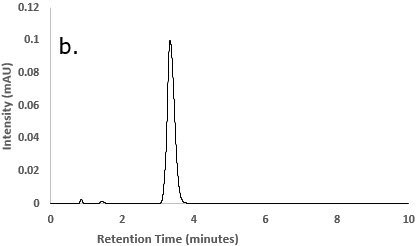
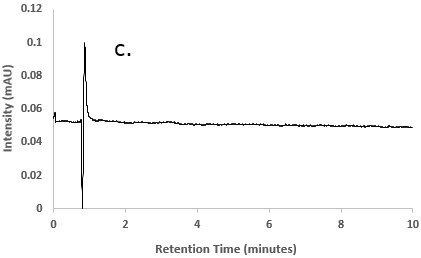
Figure 2.Chromatograms of a) Besifloxacin reference STD solution; b) Besifloxacin test solution; and c) Blank solution (diluent)
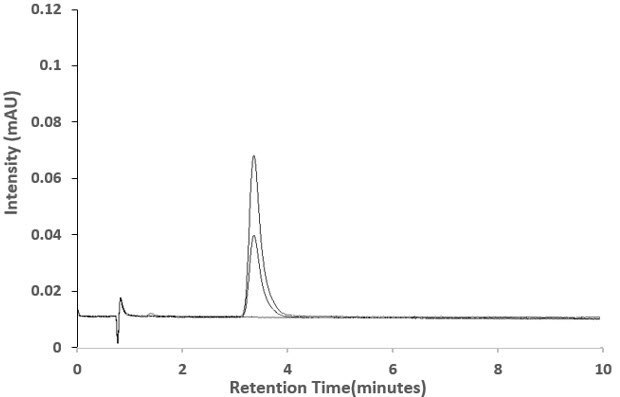
Figure 3.LOQ and LOD chromatograms of Besifloxacin overlaid with blank
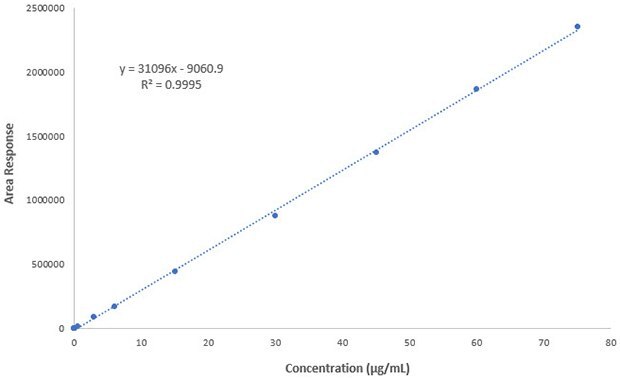
Figure 4.Linearity plot of Besifloxacin reference standard
Results and Discussion
An isocratic RP-HPLC method for the determination of Besifloxacin in ophthalmic suspension was developed using Chromolith® SpeedROD RP-18 endcapped column with UV detection at 289 nm. The experimental data indicated an excellent linearity with an r2 value of 0.999 (Figure 4) for the selected concentration range (0.005 – 75 µg/mL, Table 4). The LOD and LOQ values were estimated as 2.3 µg/mL and 7 µg/mL, respectively. The method precision for assay was below 1% RSD and the percentage recovery found to be ranging from 102.1 -104 % (Table 5).
In conclusion, the data for linearity, system suitability, repeatability of the method suggests its aptness for the assay of Besifloxacin in opthalmic suspension using Chromolith® SpeedROD RP-18 endcapped column.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?