ログインで組織・契約価格をご覧ください。
サイズを選択してください
この商品について
実験式(ヒル表記法):
C12H8S
CAS番号:
分子量:
184.26
UNSPSC Code:
41116107
NACRES:
NA.24
PubChem Substance ID:
EC Number:
205-072-9
Beilstein/REAXYS Number:
121101
MDL number:
製品名
ジベンゾチオフェン, analytical standard
InChI key
IYYZUPMFVPLQIF-UHFFFAOYSA-N
InChI
1S/C12H8S/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H
SMILES string
c1ccc2c(c1)sc3ccccc23
grade
analytical standard
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
332-333 °C (lit.)
mp
97-100 °C (lit.)
application(s)
environmental
format
neat
Quality Level
類似した製品をお探しですか? 訪問 製品比較ガイド
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Other Notes
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
signalword
Warning
hcodes
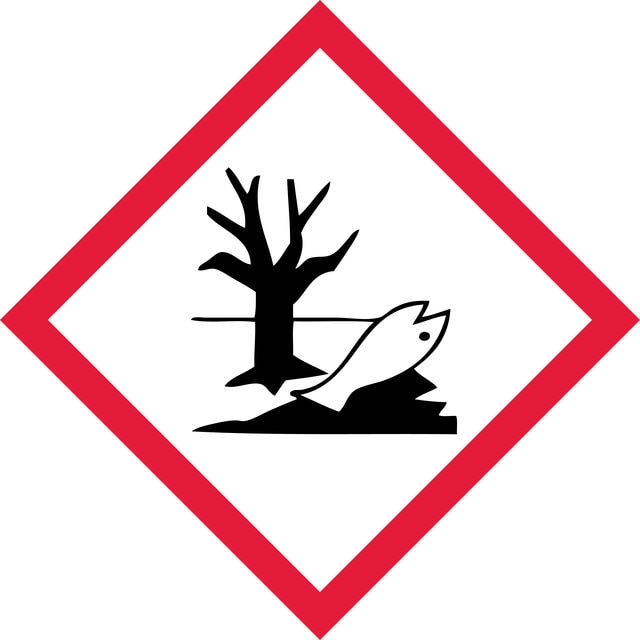
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
保管分類
11 - Combustible Solids
wgk
WGK 3
flash_point_f
338.0 °F
flash_point_c
170 °C
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Mykola Seredych et al.
ChemSusChem, 4(1), 139-147 (2011-01-13)
Adsorption of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (DMDBT) from simulated diesel fuel was investigated with polymer-derived carbon matrices. Sulfur was incorporated to the carbon surface via a high-temperature hydrogen sulfide reduction of oxygen-containing groups. The resultant carbons were characterized by nitrogen
A Stephen K Hashmi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(21), 6576-6580 (2012-04-21)
With the IPr ligand (IPr=1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene) on gold(I) excellent yields in the benzanellation of 2-substituted thiophenes, benzothiophenes, pyrroles, benzofurans, and indoles were achieved. The 1-siloxybut-3-ynyl side chains, incorporated in the anellation, are easily accessible by the addition of a propargyl metal
Santhosh Reddy Patpi et al.
Journal of medicinal chemistry, 55(8), 3911-3922 (2012-03-28)
A molecular hybridization approach is an emerging structural modification tool to design new molecules with improved pharmacophoric properties. In this study, 1,2,3-triazole-based Mycobacterium tuberculosis inhibitors and synthetic and natural product-based tricyclic (carbazole, dibenzo[b,d]furan, and dibenzo[b,d]thiophene) antimycobacterial agents were integrated in
Ellen M Cooper et al.
Environmental toxicology and chemistry, 29(11), 2409-2416 (2010-09-24)
Biodegradation of pollutants often results in incomplete mineralization and formation of degradation products with unknown chemical and toxicological characteristics. Ultraviolet (UV) irradiation, a common technology used in water and wastewater treatment, may help reduce aqueous concentrations of degradation products produced
Adeniyi S Ogunlaja et al.
Dalton transactions (Cambridge, England : 2003), 41(45), 13908-13918 (2012-10-02)
The reaction between [V(IV)OSO(4)] and the tetradentate N(2)O(2)-donor Schiff base ligand, N,N-bis(o-hydroxybenzaldehyde)phenylenediamine (sal-HBPD), obtained by the condensation of salicylaldehyde and o-phenylenediamine in a molar ratio of 2 : 1 respectively, resulted in the formation of [V(IV)O(sal-HBPD)]. The molecular structure of
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)