ログインで組織・契約価格をご覧ください。
サイズを選択してください
この商品について
化学式:
Na2SeO3
CAS番号:
分子量:
172.94
NACRES:
NA.22
PubChem Substance ID:
UNSPSC Code:
12352302
EC Number:
233-267-9
MDL number:
Assay:
≥90.0% (RT)
Form:
powder
Application
Sodium selenite can be used:
- In dehydrogenation of benzoins to benzils and, flavanones to flavones under thermal and microwave conditions.
- As a catalyst along with cysteine or glutathione in the reductive debromination of vic-dibromides to alkenes.
- To prepare bis(methylmercuric) selenide (BMS) by reacting with methylmercuric chloride and reduced glutathione or cysteine.
Sodium selenite is a common source of selenium in the synthesis of zinc selenide (ZnSe). It is used as a key precursor in the synthesis of bismuth selenide nanocomposites, nickel cobalt selenides, selenium nanobelts and t-selenium nanotubes.
signalword
Danger
Hazard Classifications
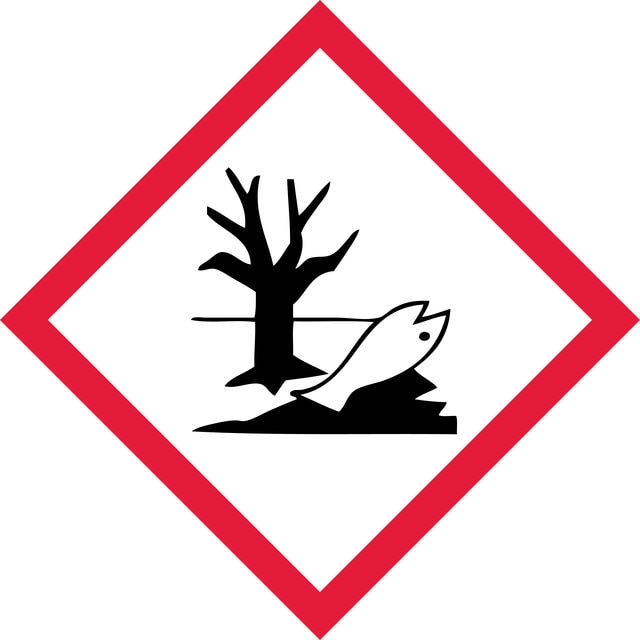
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
supp_hazards
保管分類
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物
pdsc
第一種指定化学物質
prtr
名称等を表示すべき危険物及び有害物
ishl_indicated
名称等を通知すべき危険物及び有害物
ishl_notified
71950-BULK: + 71950-RSAMPLE: + 71950-500G:4548173307084 + 71950-100G:4548173307077 + 71950-VAR:
jan
Hydrothermal synthesis and high photocatalytic activity of 3D wurtzite ZnSe hierarchical nanostructures.
Cao F, et al.
The Journal of Physical Chemistry C, 112(44), 17095-17101 (2008)
Nucleation- dissolution- recrystallization: a new growth mechanism for t-selenium Nanotubes.
Xi G, et al.
Crystal Growth & Design, 6(2), 577-582 (2006)
Cellulose-directed growth of selenium nanobelts in solution.
Lu Q, et al.
Chemistry of Materials, 18(1) (2006)
Sodium selenite-dimethylsulfoxide: a highly efficient reagent for dehydrogenation
Lamba M and Makrandi JK
J. Chem. Res. (M), 2008(4), 225-226 (2008)
Selenium-catalysed debromination of vic-dibromides to alkenes with cysteine or glutathione
Yanada, Kazuo and Yanada, Reiko and Meguri, Haruo
Journal of the Chemical Society. Chemical Communications, 2008(10), 730-732 (1990)
資料
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)