サイズを選択してください
この商品について
InChI key
FEWJPZIEWOKRBE-JCYAYHJZSA-N
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
SMILES string
O[C@H]([C@@H](O)C(O)=O)C(O)=O
grade
certified reference material, pharmaceutical secondary standard
agency
traceable to USP 1643340
vapor density
5.18 (vs air)
API family
tartaric acid
CofA
current certificate can be downloaded
autoignition temp.
797 °F
packaging
pkg of 1 g
technique(s)
HPLC: suitable, gas chromatography (GC): suitable
mp
170-172 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-30°C
Quality Level
類似した製品をお探しですか? 訪問 製品比較ガイド
General description
Application
Analysis Note
Other Notes
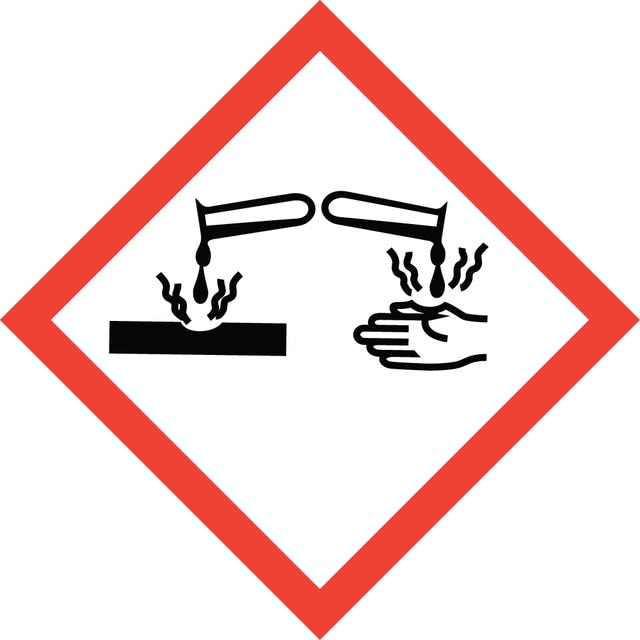
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1
保管分類
11 - Combustible Solids
wgk
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
PHR1472-1G-PW: + PHR1472-1G:
jan
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
プロトコル
Science Slam panel: Leading gene therapy developers discuss commercialization challenges and the importance of robust process development plans.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)