サイズを選択してください
この商品について
InChI key
XMEVHPAGJVLHIG-FMZCEJRJSA-N
InChI
1S/C22H24N2O8.ClH/c1-21(31)8-5-4-6-11(25)12(8)16(26)13-9(21)7-10-15(24(2)3)17(27)14(20(23)30)19(29)22(10,32)18(13)28;/h4-6,9-10,15,25,27-28,31-32H,7H2,1-3H3,(H2,23,30);1H/t9-,10-,15-,21+,22-;/m0./s1
SMILES string
Cl.CN(C)[C@H]1[C@@H]2C[C@H]3C(=C(O)[C@]2(O)C(=O)C(C(N)=O)=C1O)C(=O)c4c(O)cccc4[C@@]3(C)O
agency
USP/NF, meets USP testing specifications
assay
≥900 (Units in µg/mg)
form
crystalline powder
optical activity
[α]/D -255 to 240° (Specific rotation )
storage condition
(Keep container tightly closed in a dry and well-ventilated place. Light sensitive.)
impurities
≤4.0%
color
yellow
mp
220-223 °C (lit.)
antibiotic activity spectrum
Gram-negative bacteria, Gram-positive bacteria
application(s)
pharmaceutical (small molecule)
mode of action
protein synthesis | interferes
storage temp.
−20°C
Quality Level
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
General description
Application
Biochem/physiol Actions
耐性の機序:細胞壁の浸透性が消失することで効果は不活性化されます。
抗菌性スペクトル:グラム陽性およびグラム陰性細菌に対し広範囲な抗菌活性があります。
Preparation Note
Other Notes
signalword
Warning
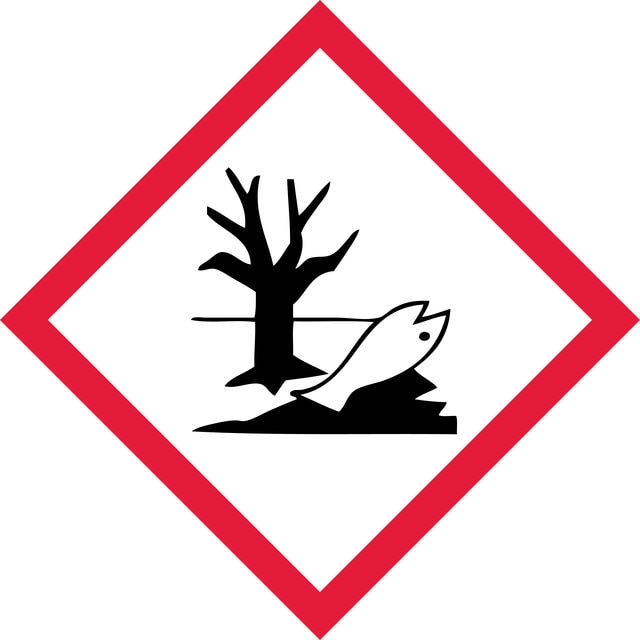
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
保管分類
11 - Combustible Solids
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
T4062-5G: + T4062-1G: + T4062-BULK: + T4062-VAR:
jan
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)