サイズを選択してください
この商品について
製品名
Dapagliflozin, ≥98% (HPLC)
InChI
1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1
SMILES string
CCOC1=CC=C(C=C1)CC2=C(C=CC([C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)=C2)Cl
InChI key
JVHXJTBJCFBINQ-ADAARDCZSA-N
assay
≥98% (HPLC)
form
powder
optical activity
[α]/D (+7.0° to +13.0°, c = 0.2 in methanol)
color
white to beige
solubility
DMSO: 2 mg/mL, clear
storage temp.
−20°C
Quality Level
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
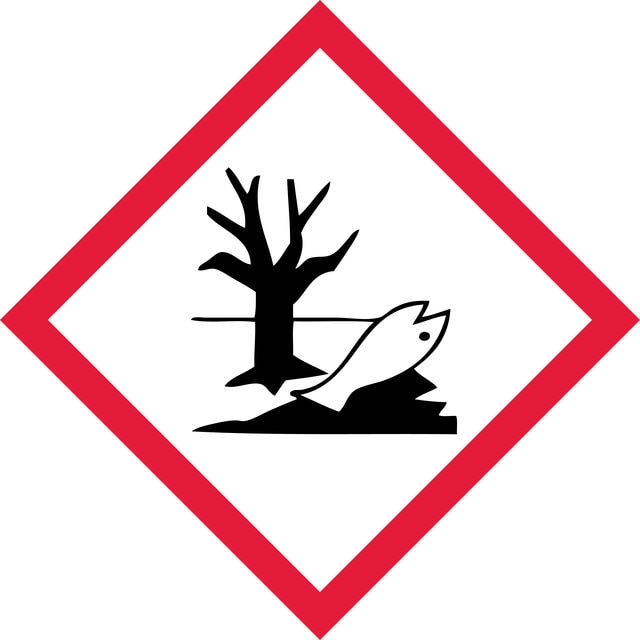
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - STOT RE 1
target_organs
Kidney
保管分類
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
SML2804-BULK: + SML2804-5MG: + SML2804-25MG: + SML2804-VAR:
jan
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)