サイズを選択してください
この商品について
InChI
1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)
SMILES string
[F2C(F2C)13F3C]C(O)=O
InChI key
QTBSBXVTEAMEQO-UHFFFAOYSA-N
grade
pharmaceutical primary standard
vapor density
2.07 (vs air)
API family
glacial acetic acid
form
liquid
autoignition temp.
800 °F
expl. lim.
16 %, 92 °F, 4 %, 59 °F
manufacturer/tradename
USP
technique(s)
gas chromatography (GC): suitable
refractive index
n20/D 1.371 (lit.)
bp
117-118 °C (lit.)
mp
16.2 °C (lit.)
density
1.04 g/mL at 25 °C (lit.)
application(s)
USP Biologics
pharmaceutical (small molecule)
format
neat
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
General description
Glacial acetic acid is a commonly used solvent and pH modifier in peptide synthesis and purification. It may be present as a residual process reagent. The USP standard is used to quantify acetic acid levels in peptide APIs and assess formulation stability.
The USP biologics peptides category encompasses a diverse range of therapeutic peptides that are essential in managing various medical conditions. These peptides, typically consisting of amino acid sequences of 40 residues or less, are critical for the development of high-quality medicines. The USP provides comprehensive standards, reference materials, and analytical procedures to ensure the identity, quality, purity, and consistency of peptide therapeutics throughout their lifecycle.
The United States Pharmacopeia (USP) provides quality standards for biologics to ensure their safety, efficacy, and quality throughout the manufacturing process. These standards assist manufacturers in adhering to regulatory requirements and help safeguard public health by reducing risks associated with biologics.
Application
詳細については、USP(米国薬局方)のモノグラフ、テリパラチド、USPNF 2021 ISSUE 6741を参照してください。
Other Notes
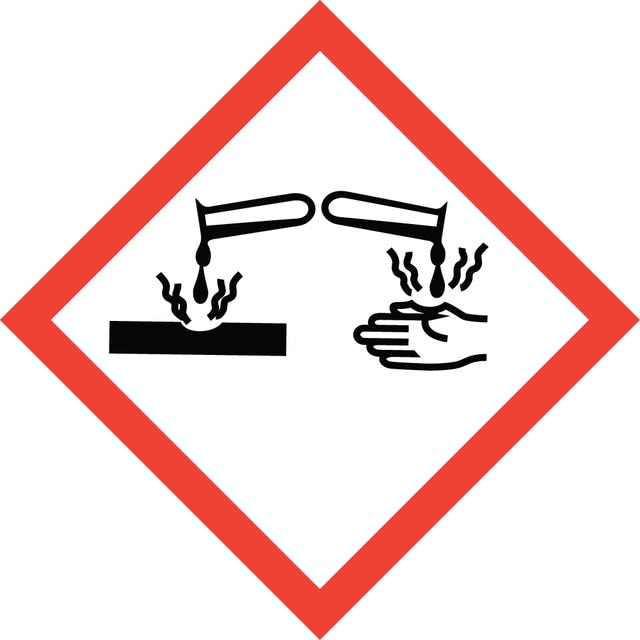
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
保管分類
3 - Flammable liquids
flash_point_f
102.2 °F - closed cup
flash_point_c
39 °C - closed cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
第4類:引火性液体 + 第二石油類 + 危険等級III + 水溶性液体
fsl
名称等を表示すべき危険物及び有害物
ishl_indicated
名称等を通知すべき危険物及び有害物
ishl_notified
1005706-3X1.5ML:4548173359243
jan
プロトコル
Science Slam panel: Leading gene therapy developers discuss commercialization challenges and the importance of robust process development plans.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)