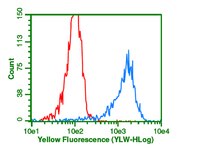
Differences in long-term memory stability and AmCREB level between forward and backward conditioned honeybees (Apis mellifera).
Felsenberg, J; Dyck, Y; Feige, J; Ludwig, J; Plath, JA; Froese, A; Karrenbrock, M; Nölle, A; Heufelder, K; Eisenhardt, D
Frontiers in behavioral neuroscience
9
91
2015
Show Abstract
In classical conditioning a predictive relationship between a neutral stimulus (conditioned stimulus; CS) and a meaningful stimulus (unconditioned stimulus; US) is learned when the CS precedes the US. In backward conditioning the sequence of the stimuli is reversed. In this situation animals might learn that the CS signals the end or the absence of the US. In honeybees 30 min and 24 h following backward conditioning a memory for the excitatory and inhibitory properties of the CS could be retrieved, but it remains unclear whether a late long-term memory is formed that can be retrieved 72 h following backward conditioning. Here we examine this question by studying late long-term memory formation in forward and backward conditioning of the proboscis extension response (PER). We report a difference in the stability of memory formed upon forward and backward conditioning with the same number of conditioning trials. We demonstrate a transcription-dependent memory 72 h after forward conditioning but do not observe a 72 h memory after backward conditioning. Moreover we find that protein degradation is differentially involved in memory formation following these two conditioning protocols. We report differences in the level of a transcription factor, the cAMP response element binding protein (CREB) known to induce transcription underlying long-term memory formation, following forward and backward conditioning. Our results suggest that these alterations in CREB levels might be regulated by the proteasome. We propose that the differences observed are due to the sequence of stimulus presentation between forward and backward conditioning and not to differences in the strength of the association of both stimuli. | | 25964749
 |
Stability of structured Kaposi's sarcoma-associated herpesvirus ORF57 protein is regulated by protein phosphorylation and homodimerization.
Majerciak, V; Pripuzova, N; Chan, C; Temkin, N; Specht, SI; Zheng, ZM
Journal of virology
89
3256-74
2015
Show Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) ORF57 plays an essential role in KSHV lytic infection by promoting viral gene expression at the posttranscriptional level. Using bioinformatic and biochemical approaches, we determined that ORF57 contains two structurally and functionally distinct domains: a disordered nonstructural N-terminal domain (amino acids [aa] 1 to 152) and a structured α-helix-rich C-terminal domain (aa 153 to 455). The N-terminal domain mediates ORF57 interaction with several RNA-protein complexes essential for ORF57 to function. The N-terminal phosphorylation by cellular casein kinase II (CKII) at S21, T32, and S43, and other cellular kinases at S95 and S97 residues in proximity of the caspase-7 cleavage site, 30-DETD-33, inhibits caspase-7 digestion of ORF57. The structured C-terminal domain mediates homodimerization of ORF57, and the critical region for this function was mapped carefully to α-helices 7 to 9. Introduction of point mutations into α-helix 7 at ORF57 aa 280 to 299, a region highly conserved among ORF57 homologues from other herpesviruses, inhibited ORF57 homodimerization and led to proteasome-mediated degradation of ORF57 protein. Thus, homodimerization of ORF57 via its C terminus prevents ORF57 from degrading and allows two structure-free N termini of the dimerized ORF57 to work coordinately for host factor interactions, leading to productive KSHV lytic infection and pathogenesis.KSHV is a human oncogenic virus linked to the development of several malignancies. KSHV-mediated oncogenesis requires both latent and lytic infection. The KSHV ORF57 protein is essential for KSHV lytic replication, as it regulates the expression of viral lytic genes at the posttranscriptional level. This report provides evidence that the structural conformation of the ORF57 protein plays a critical role in regulation of ORF57 stability. Phosphorylation by CKII on the identified serine/threonine residues at the N-terminal unstructured domain of ORF57 prevents its digestion by caspase-7. The C-terminal domain of ORF57, which is rich in α-helices, contributes to homodimerization of ORF57 to prevent proteasome-mediated protein degradation. Elucidation of the ORF57 structure not only enables us to better understand ORF57 stability and functions but also provides an important tool for us to modulate ORF57's activity with the aim to inhibit KSHV lytic replication. | | 25568207
 |
Coupled local translation and degradation regulate growth cone collapse.
Deglincerti, A; Liu, Y; Colak, D; Hengst, U; Xu, G; Jaffrey, SR
Nature communications
6
6888
2015
Show Abstract
Local translation mediates axonal responses to Semaphorin3A (Sema3A) and other guidance cues. However, only a subset of the axonal proteome is locally synthesized, whereas most proteins are trafficked from the soma. The reason why only specific proteins are locally synthesized is unknown. Here we show that local protein synthesis and degradation are linked events in growth cones. We find that growth cones exhibit high levels of ubiquitination and that local signalling pathways trigger the ubiquitination and degradation of RhoA, a mediator of Sema3A-induced growth cone collapse. Inhibition of RhoA degradation is sufficient to remove the protein-synthesis requirement for Sema3A-induced growth cone collapse. In addition to RhoA, we find that locally translated proteins are the main targets of the ubiquitin-proteasome system in growth cones. Thus, local protein degradation is a major feature of growth cones and creates a requirement for local translation to replenish proteins needed to maintain growth cone responses. | | 25901863
 |
Pharmacological targeting of valosin containing protein (VCP) induces DNA damage and selectively kills canine lymphoma cells.
Nadeau, MÈ; Rico, C; Tsoi, M; Vivancos, M; Filimon, S; Paquet, M; Boerboom, D
BMC cancer
15
479
2015
Show Abstract
Valosin containing protein (VCP) is a critical mediator of protein homeostasis and may represent a valuable therapeutic target for several forms of cancer. Overexpression of VCP occurs in many cancers, and often in a manner correlating with malignancy and poor outcome. Here, we analyzed VCP expression in canine lymphoma and assessed its potential as a therapeutic target for this disease.VCP expression in canine lymphomas was evaluated by immunoblotting and immunohistochemistry. The canine lymphoma cell lines CLBL-1, 17-71 and CL-1 were treated with the VCP inhibitor Eeyarestatin 1 (EER-1) at varying concentrations and times and were assessed for viability by trypan blue exclusion, apoptosis by TUNEL and caspase activity assays, and proliferation by propidium iodide incorporation and FACS. The mechanism of EER-1 action was determined by immunoblotting and immunofluorescence analyses of Lys48 ubiquitin and markers of ER stress (DDIT3), autophagy (SQSTM1, MAP1LC3A) and DNA damage (γH2AFX). TRP53/ATM-dependent signaling pathway activity was assessed by immunoblotting for TRP53 and phospho-TRP53 and real-time RT-PCR measurement of Cdkn1a mRNA.VCP expression levels in canine B cell lymphomas were found to increase with grade. EER-1 treatment killed canine lymphoma cells preferentially over control peripheral blood mononuclear cells. EER-1 treatment of CLBL-1 cells was found to both induce apoptosis and cell cycle arrest in G1. Unexpectedly, EER-1 did not appear to act either by inducing ER stress or inhibiting the aggresome-autophagy pathway. Rather, a rapid and dramatic increase in γH2AFX expression was noted, indicating that EER-1 may act by promoting DNA damage accumulation. Increased TRP53 phosphorylation and Cdkn1a mRNA levels indicated an activation of the TRP53/ATM DNA damage response pathway in response to EER-1, likely contributing to the induction of apoptosis and cell cycle arrest.These results correlate VCP expression with malignancy in canine B cell lymphoma. The selective activity of EER-1 against lymphoma cells suggests that VCP will represent a clinically useful therapeutic target for the treatment of lymphoma. We further suggest a mechanism of EER-1 action centered on the DNA repair response that may be of central importance for the design and characterization of VCP inhibitory compounds for therapeutic use. | | 26104798
 |
Endocytic Adaptor Protein Tollip Inhibits Canonical Wnt Signaling.
Toruń, A; Szymańska, E; Castanon, I; Wolińska-Nizioł, L; Bartosik, A; Jastrzębski, K; Miętkowska, M; González-Gaitán, M; Miaczynska, M
PloS one
10
e0130818
2015
Show Abstract
Many adaptor proteins involved in endocytic cargo transport exhibit additional functions in other cellular processes which may be either related to or independent from their trafficking roles. The endosomal adaptor protein Tollip is an example of such a multitasking regulator, as it participates in trafficking and endosomal sorting of receptors, but also in interleukin/Toll/NF-κB signaling, bacterial entry, autophagic clearance of protein aggregates and regulation of sumoylation. Here we describe another role of Tollip in intracellular signaling. By performing a targeted RNAi screen of soluble endocytic proteins for their additional functions in canonical Wnt signaling, we identified Tollip as a potential negative regulator of this pathway in human cells. Depletion of Tollip potentiates the activity of β-catenin/TCF-dependent transcriptional reporter, while its overproduction inhibits the reporter activity and expression of Wnt target genes. These effects are independent of dynamin-mediated endocytosis, but require the ubiquitin-binding CUE domain of Tollip. In Wnt-stimulated cells, Tollip counteracts the activation of β-catenin and its nuclear accumulation, without affecting its total levels. Additionally, under conditions of ligand-independent signaling, Tollip inhibits the pathway after the stage of β-catenin stabilization, as observed in human cancer cell lines, characterized by constitutive β-catenin activity. Finally, the regulation of Wnt signaling by Tollip occurs also during early embryonic development of zebrafish. In summary, our data identify a novel function of Tollip in regulating the canonical Wnt pathway which is evolutionarily conserved between fish and humans. Tollip-mediated inhibition of Wnt signaling may contribute not only to embryonic development, but also to carcinogenesis. Mechanistically, Tollip can potentially coordinate multiple cellular pathways of trafficking and signaling, possibly by exploiting its ability to interact with ubiquitin and the sumoylation machinery. | | 26110841
 |
TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING.
Wang, Y; Lian, Q; Yang, B; Yan, S; Zhou, H; He, L; Lin, G; Lian, Z; Jiang, Z; Sun, B
PLoS pathogens
11
e1005012
2015
Show Abstract
Uncontrolled immune responses to intracellular DNA have been shown to induce autoimmune diseases. Homeostasis regulation of immune responses to cytosolic DNA is critical for limiting the risk of autoimmunity and survival of the host. Here, we report that the E3 ubiquitin ligase tripartite motif protein 30α (TRIM30α) was induced by herpes simplex virus type 1 (HSV-1) infection in dendritic cells (DCs). Knockdown or genetic ablation of TRIM30α augmented the type I IFNs and interleukin-6 response to intracellular DNA and DNA viruses. Trim30α-deficient mice were more resistant to infection by DNA viruses. Biochemical analyses showed that TRIM30α interacted with the stimulator of interferon genes (STING), which is a critical regulator of the DNA-sensing response. Overexpression of TRIM30α promoted the degradation of STING via K48-linked ubiquitination at Lys275 through a proteasome-dependent pathway. These findings indicate that E3 ligase TRIM30α is an important negative-feedback regulator of innate immune responses to DNA viruses by targeting STING. | | 26114947
 |
Sirt1-deficiency causes defective protein quality control.
Tomita, T; Hamazaki, J; Hirayama, S; McBurney, MW; Yashiroda, H; Murata, S
Scientific reports
5
12613
2015
Show Abstract
Protein quality control is an important mechanism to maintain cellular homeostasis. Damaged proteins have to be restored or eliminated by degradation, which is mainly achieved by molecular chaperones and the ubiquitin-proteasome system. The NAD(+)-dependent deacetylase Sirt1 has been reported to play positive roles in the regulation of cellular homeostasis in response to various stresses. However, its contribution to protein quality control remains unexplored. Here we show that Sirt1 is involved in protein quality control in both an Hsp70-dependent and an Hsp70-independent manner. Loss of Sirt1 led to the accumulation of ubiquitinated proteins in cells and tissues, especially upon heat stress, without affecting proteasome activities. This was partly due to decreased basal expression of Hsp70. However, this accumulation was only partially alleviated by overexpression of Hsp70 or induction of Hsp70 upon heat shock in Sirt1-deficient cells and tissues. These results suggest that Sirt1 mediates both Hsp70-dependent and Hsp70-independent protein quality control. Our findings cast new light on understanding the role of Sirt1 in maintaining cellular homeostasis. | | 26219988
 |
Cell fate decisions regulated by K63 ubiquitination of tumor necrosis factor receptor 1.
Fritsch, J; Stephan, M; Tchikov, V; Winoto-Morbach, S; Gubkina, S; Kabelitz, D; Schütze, S
Molecular and cellular biology
34
3214-28
2014
Show Abstract
Signaling by tumor necrosis factor (TNF) receptor 1 (TNF-R1), a prototypic member of the death receptor family, mediates pleiotropic biological outcomes ranging from inflammation and cell proliferation to cell death. Although many elements of specific signaling pathways have been identified, the main question of how these selective cell fate decisions are regulated is still unresolved. Here we identified TNF-induced K63 ubiquitination of TNF-R1 mediated by the ubiquitin ligase RNF8 as an early molecular checkpoint in the regulation of the decision between cell death and survival. Downmodulation of RNF8 prevented the ubiquitination of TNF-R1, blocked the internalization of the receptor, prevented the recruitment of the death-inducing signaling complex and the activation of caspase-8 and caspase-3/7, and reduced apoptotic cell death. Conversely, recruitment of the adaptor proteins TRADD, TRAF2, and RIP1 to TNF-R1, as well as activation of NF-κB, was unimpeded and cell growth and proliferation were significantly enhanced in RNF8-deficient cells. Thus, K63 ubiquitination of TNF-R1 can be sensed as a new level of regulation of TNF-R1 signaling at the earliest stage after ligand binding. | | 24980434
 |
Cellular inhibitor of apoptosis (cIAP)-mediated ubiquitination of phosphofurin acidic cluster sorting protein 2 (PACS-2) negatively regulates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) cytotoxicity.
Guicciardi, ME; Werneburg, NW; Bronk, SF; Franke, A; Yagita, H; Thomas, G; Gores, GJ
PloS one
9
e92124
2014
Show Abstract
Lysosomal membrane permeabilization is an essential step in TRAIL-induced apoptosis of liver cancer cell lines. TRAIL-induced lysosomal membrane permeabilization is mediated by the multifunctional sorting protein PACS-2 and repressed by the E3 ligases cIAP-1 and cIAP-2. Despite the opposing roles for PACS-2 and cIAPs in TRAIL-induced apoptosis, an interaction between these proteins has yet to be examined. Herein, we report that cIAP-1 and cIAP-2 confer TRAIL resistance to hepatobiliary cancer cell lines by reducing PACS-2 levels. Under basal conditions, PACS-2 underwent K48-linked poly-ubiquitination, resulting in PACS-2 proteasomal degradation. Biochemical assays showed cIAP-1 and cIAP-2 interacted with PACS-2 in vitro and co-immunoprecipitation studies demonstrated that the two cIAPs bound PACS-2 in vivo. More importantly, both cIAP-1 and cIAP-2 directly mediated PACS-2 ubiquitination in a cell-free assay. Single c-Iap-1 or c-Iap-2 gene knock-outs in mouse hepatocytes did not lead to PACS-2 accumulation. However, deletion of both cIAP-1 and cIAP-2 reduced PACS-2 ubiquitination, which increased PACS-2 levels and sensitized HuH-7 cells to TRAIL-induced lysosomal membrane permeabilization and apoptosis. Correspondingly, deletion of cIAPs sensitized wild-type, but not PACS-2-deficient hepatocarcinoma cells or Pacs-2-/- mouse hepatocytes to TRAIL-induced apoptosis. Together, these data suggest cIAPs constitutively downregulate PACS-2 by polyubiquitination and proteasomal degradation, thereby restraining TRAIL-induced killing of liver cancer cells. | | 24633224
 |
Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species.
Scotter, EL; Vance, C; Nishimura, AL; Lee, YB; Chen, HJ; Urwin, H; Sardone, V; Mitchell, JC; Rogelj, B; Rubinsztein, DC; Shaw, CE
Journal of cell science
127
1263-78
2014
Show Abstract
TAR DNA-binding protein (TDP-43, also known as TARDBP) is the major pathological protein in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Large TDP-43 aggregates that are decorated with degradation adaptor proteins are seen in the cytoplasm of remaining neurons in ALS and FTD patients post mortem. TDP-43 accumulation and ALS-linked mutations within degradation pathways implicate failed TDP-43 clearance as a primary disease mechanism. Here, we report the differing roles of the ubiquitin proteasome system (UPS) and autophagy in the clearance of TDP-43. We have investigated the effects of inhibitors of the UPS and autophagy on the degradation, localisation and mobility of soluble and insoluble TDP-43. We find that soluble TDP-43 is degraded primarily by the UPS, whereas the clearance of aggregated TDP-43 requires autophagy. Cellular macroaggregates, which recapitulate many of the pathological features of the aggregates in patients, are reversible when both the UPS and autophagy are functional. Their clearance involves the autophagic removal of oligomeric TDP-43. We speculate that, in addition to an age-related decline in pathway activity, a second hit in either the UPS or the autophagy pathway drives the accumulation of TDP-43 in ALS and FTD. Therapies for clearing excess TDP-43 should therefore target a combination of these pathways. | | 24424030
 |