조직 및 계약 가격을 보려면 로그인를 클릭합니다.
크기 선택
제품정보 (DICE 배송 시 비용 별도)
Linear Formula:
CH3CO2H
CAS 번호:
Molecular Weight:
60.05
UNSPSC Code:
12352106
NACRES:
NA.21
PubChem Substance ID:
EC Number:
200-580-7
Beilstein/REAXYS Number:
506007
MDL number:
Assay:
≥99%
Form:
liquid
InChI key
QTBSBXVTEAMEQO-UHFFFAOYSA-N
InChI
1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)
SMILES string
[F2C(F2C)13F3C]C(O)=O
vapor density
2.07 (vs air)
product line
ReagentPlus®
assay
≥99%
form
liquid
autoignition temp.
800 °F
expl. lim.
16 %, 92 °F, 4 %, 59 °F
refractive index
n20/D 1.371 (lit.)
pH
2.5 (20 °C, 50 g/L)
bp
117-118 °C (lit.)
mp
16.2 °C (lit.)
solubility
alcohol: miscible(lit.), carbon disulfide: insoluble(lit.), glycerol: miscible(lit.), water: miscible(lit.)
density
1.04 g/mL at 25 °C (lit.)
storage temp.
room temp
Quality Level
유사한 제품을 찾으십니까? 방문 제품 비교 안내
General description
Acetic acid is an aliphatic organic acid. It is a hygroscopic, corrosive liquid with a vinegar-like odor. It can be synthesized by oxidizing acetaldehyde in the presence of manganese or cobalt salts. It is utilized for synthesizing acetic anhydride, cellulose acetate and acetic esters. Its impact on the degradation of historic paper has been analyzed.
Application
Acetic acid (AcOH) can be used as:
Acetic acid can also be used in the following:
- A reaction solvent in many organic reactions such as bromination, hydrolysis, solvolysis, reductions, and hydrogenations.
- A reagent in the protonolysis of organometallic compounds.
- An acetylating agent for the acetylation of electron-rich aromatic compounds.
- A catalyst to synthesize di(indolyl)methanes by the condensation reaction of indole and aromatic aldehydes.
- A solvent system to prepare 3,4-dihydropyrimidin-2(1H)-one derivative via Biginelli reaction of aromatic aldehydes, 1,3-dicarbonyl compounds, and urea in the presence of a boric acid catalyst.
Acetic acid can also be used in the following:
- Manganese(III) acetate/AcOH catalytic system is used in the conversion of alkenes to lactones.
- Iron salts/AcOH is used to oxidize 2-methylnaphthalene to 2-methyl-1-naphthol in the presence of H2O2.
Features and Benefits
- Good stability towards many reagents
- Excellent solubility of organic compounds in this reagent
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Danger
hcodes
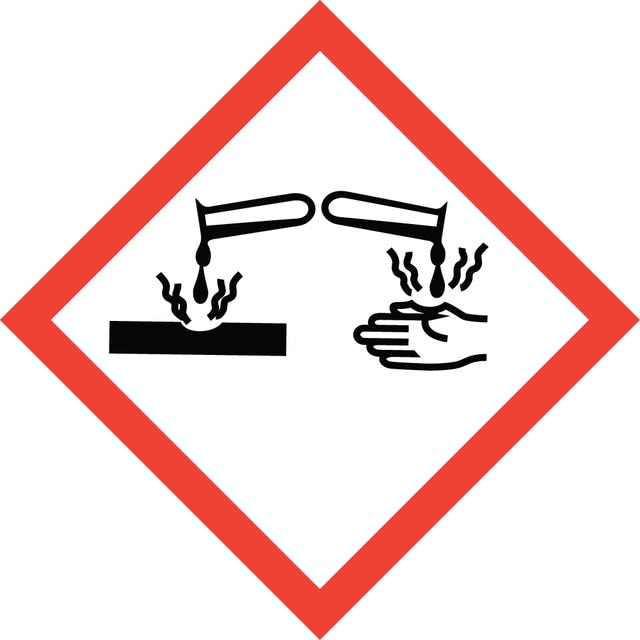
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
저장 등급
3 - Flammable liquids
wgk
WGK 1
flash_point_f
102.2 °F - closed cup
flash_point_c
39 °C - closed cup
Ash M and Ash I.
Handbook of Preservatives, 266-266 (2004)
Effects of NO2 and acetic acid on the stability of historic paper.
Menart Eet al.
Cellulose, 21(5), 3701-3713 (2014)
Maize Kernels-Fixation in FAA, Embedding, Sectioning and Feulgen Staining.
Kladnik A.
Bio-protocol, 3(15), e835-e835 (2013)
Thermooxidative aging of polydicyclopentadiene in glassy state.
Richaud E, et al.
Polymer Degradation and Stability, 102, 95-104 (2014)
Eagleson M.
Concise Encyclopedia Chemistry, 5-5 (1994)
프로토콜
Preparation of Plasmid DNA by Alkaline Lysis with SDS: Maxipreparation between Cold Spring Harbor Laboratory Press and our research team.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.